Publications 2025
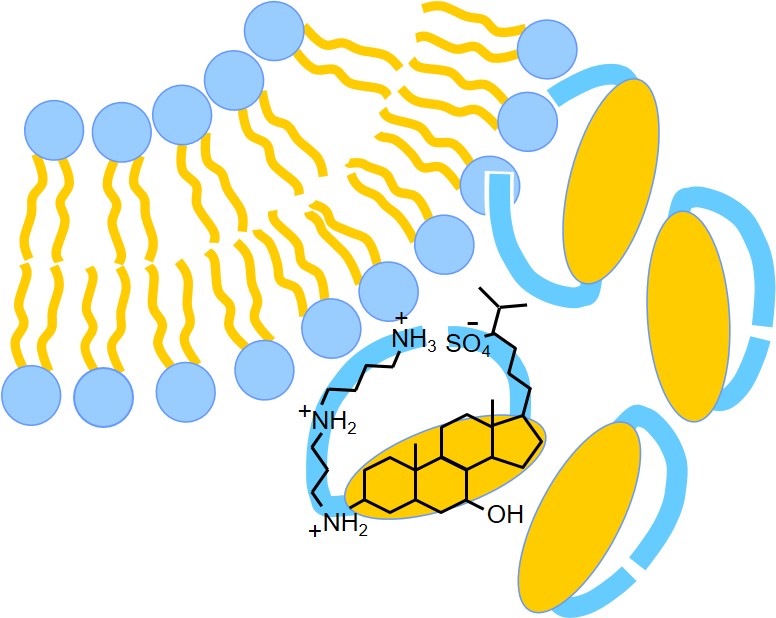 S.L. Grage, N. Guschtschin-Schmidt, B. Meng, A. Kohlmeyer, S. Afonin, A.S. Ulrich. Interaction of squalamine with lipid membranes. J. Chem. Phys. B, in press (2025)
S.L. Grage, N. Guschtschin-Schmidt, B. Meng, A. Kohlmeyer, S. Afonin, A.S. Ulrich. Interaction of squalamine with lipid membranes. J. Chem. Phys. B, in press (2025)
DOI: 10.1021/acs.jcpb.4c06576
G. Mussabek, S. Baktygerey, Y. Taurbayev, D. Yermukhamed, N. Zhylkybayeva, A.N. Zaderko, V.E. Diyuk, S. Afonin, L.M. Grishchenko, V. V. Lisnyak. Gas-phase approach to the modification of carbon surfaces by F-, Cl- and Br-containing groups and their selective substitution for catalytic sulfo groups. Appl. Surf. Sci. 690, 162285 (2025)
DOI: 10.1016/j.apsusc.2024.162285
E. S trandberg, P. Horten, D. Bentz, P. Wadhwani, J. Bürck, A.S.Ulrich. Trp residues near peptide termini enhance the pore-forming activity of cationic amphipathic α-helices. Biophys. Chem. 318, 107365 (2025)
trandberg, P. Horten, D. Bentz, P. Wadhwani, J. Bürck, A.S.Ulrich. Trp residues near peptide termini enhance the pore-forming activity of cationic amphipathic α-helices. Biophys. Chem. 318, 107365 (2025)
DOI: 10.1016/j.bpc.2024.107365
Publications 2024
M. Garcia-Chame, P. Wadhwani, J. Pfeifer, U. Schepers, C.M. Niemeyer and C.M. Dominguez. A versatile microfluidic platform for extravasation studies based on DNA origami-cell interactions. Angew. Chem. Int. Ed. 63, e202318805 (2024)
DOI: 10.1002/anie.202318805
A. Hrebonkin, S. Afonin, A. Nikitjuka, O.V. Borysov, G. Leitis, O. Babii, S. Koniev, T. Lorig, S.L. Grage, P. Nick, A.S. Ulrich, A. Jirgensons, I.V. Komarov. Spiropyran-based photoisomerizable α-amino acid for membrane-active peptide modification. Chem. Eur. J. 30, e202400066 (2024)
DOI:10.1002/chem.202400066
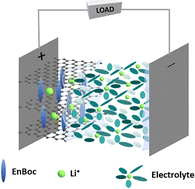 S. Mandal, Vijayamohanan.K. Pillai, M.R. Ponraj, Thushara K M, Jebasingh Bhagavathsingh, S.L. Grage, X. Peng, J.W. Kang, D. Liepmann, A.N.M. Kannan, V. Thavasi, Venkatesan Renugopalakrishnan. van der Waals gap modulation of graphene oxide through mono-Boc ethylenediamine anchoring for superior Li-ion batteries. Energy Adv. 3, 1977–1991 (2024)
S. Mandal, Vijayamohanan.K. Pillai, M.R. Ponraj, Thushara K M, Jebasingh Bhagavathsingh, S.L. Grage, X. Peng, J.W. Kang, D. Liepmann, A.N.M. Kannan, V. Thavasi, Venkatesan Renugopalakrishnan. van der Waals gap modulation of graphene oxide through mono-Boc ethylenediamine anchoring for superior Li-ion batteries. Energy Adv. 3, 1977–1991 (2024)
DOI: 10.1039/d4ya00217b
G. Mussabek, S. Baktygerey, Y. Taurbayev, D. Yermukhamed, N. Zhylkybayeva, V.E. Diyuk, A. Zaderko, S. Afonin, R. Mariychuk, M. Kaňuchová, V.V. Lisnyak. Fluorine and bromine dual-doped nanoporous carbons: Preparation and surface chemistry studies. ACS Omega 9, 38618-38628 (2024)
DOI: 10.1021/acsomega.4c04179
G. Mussabek, S. Baktygerey, Y. Taurbayev, D. Yermukhamed, N. Zhylkybayeva, A.N. Zaderko, V.E. Diyuk, S. Afonin, G. Yar-Mukhamedova, R.T. Mariychuk, L.M. Grishchenko, M. Kaňuchová, V.V. Lisnyak. Surface chemistry and catalytic activity in HO decomposition of pyrolytically fluoralkylated activated carbons. RSC Adv. 14, 29052–29071 (2024)
DOI: 10.1039/d4ra04883k
I. Rudnik-Jansen, J. Du, N. Karssemakers-Degen, A.R. Tellegen, P. Wadhwani, D. Zuncheddu, B.P. Meij, J. Thies, P. Emans, F.C. Öner, G. Mihov, J.P. Garcia, A.S. Ulrich, S. Grad, M.A. Tryfonidou, H. van Ingen, L.B. Creemers. Drug retention after intradiscal administration. Drug Discovery 31, 2415579 (2024)
DOI: 10.1080/10717544.2024.2415579
Y. Tian, M. Seifermann, L. Bauer, C. Luchena, J.J. Wiedmann, S. Schmidt, A. Geisel, S. Afonin, J. Höpfner, M. Brehm, X. Liu, C. Hopf, A.A. Popova, P.A. Levkin. High-throughput miniaturized synthesis of PROTAC-like molecules. Small 20, e2307215 (2024)
DOI:10.1002/smll.202307215
A.S.Ulrich. No nanobots in vaccines - just lipids on the loose: Commentary on Lee and Broudy (2024), “Real-time self-assembly of stereomicroscopically visible artificial constructs in incubated specimens of mRNA products mainly from Pfizer and Moderna: A comprehensive longitundinal study”. Intl. J. Vaccine Theory Practice Research 3, 1244.1-10 (2024)
DOI: 10.56098/7hsjff81
S. Wörner, P. Rauthe, J. Werner, S. Afonin, A.S. Ulrich, A.N. Unterreiner, H.A. Wagenknecht. Flavin-induced charge separation in transmembrane model peptides. Org. Biomol. Chem. 22, 5930-5935 (2024)
DOI:10.1039/d4ob00932k.
Publications 2023
H.G. Abernathy, J. Saha, L.K. Kemp, P. Wadhwani, T.D. Clemons, S.E. Morgan, V. Rangachari. De novo amyloid peptides with subtle sequence variations differ in their self-assembly and nanomechanical properties. Soft Matter 19, 5150-5159 (2023)
DOI: 10.1039/d3sm00604b
D. Gauder, A. Bott, J. Gölz, G. Lanza. Simulation uncertainty determination of single flank rolling tests using monte carlo simulation and skin model shapes for zero defect manufacturing of micro gears. Computers in Industry 146, 103854 (2023)
DOI: 10.1016/j.compind.2023.103854
F. Greil, R. Punampalam, T.H. Walther, S. Heißler, A.S. Ulrich. "Iteratively nafionated water” in its solid phase at room temperature is in fact a mixture of lyophilized biological and non-biological contaminants. J. Mol. Liquids 385, 122351 (2023)
DOI: 10.1016/j.molliq.2023.122351
K. Horbatok, T. Makhnii, V. Kosach, V. Danko, A. Kovalenko, S. Fatiushchenkov, P. Borysko, I. Pishel, O. Babii, A.S. Ulrich, T. Schober, S. Afonin, I.V. Komarov. In vitro and in vivo evaluation of photocontrolled biologically active compounds - potential drug candidates for cancer photopharmacology. J. Vis. Exp. 199, e64902 (2023)
DOI:10.3791/64902
R. Leibe, S. Fritsch-Decker, F. Gussmann, A.M. Wagbo, P. Wadhwani, S. Diabaté, W. Wenzel, A.S. Ulrich, C. Weiss. Key Role of choline head groups in large unilamellar phospholipid vesicles for the interaction with and rupture by silica nanoparticles. Small 2207593 (2023)
DOI: 10.1002/smll.202207593
C. Li, H. Schopmans, L. Langer, S. Marschner, A. Chandresh, J. Bürck, Y. Tsuchiya, A. Chihaya, W. Wenzel, S. Bräse, M. Kozlowska, L. Heinke. Twisting of porphyrin by assembly in a metal‐organic framework yielding chiral photoconducting films for circularly‐polarized‐light detection. Angew. Chem. Intl. Ed. 62, e202217377 (2023)
DOI: 10.1002/anie.202217377
M. Nishimura, Y. Kawaguchi, K. Kuroki, Y. Nakagawa, T. Masuda, T. Sakai, K. Kawano, H. Hirose, M. Imanishi, T. Takatani-Nakase, S. Afonin, A.S. Ulrich, S. Futaki. Structural dissection of Epsin‐1 N‐terminal helical peptide: The role of hydrophobic residues in modulating membrane curvature. Chemistry 29, e20230129 (2023)
DOI: 10.1002/chem.202300129
E.R. Stockwald, L.M.E. Steger, S. Vollmer, C. Gottselig, S.L. Grage, J. Bürck, S. Afonin, J. Fröbel, A.-S. Blümmel, J. Setzler, W. Wenzel, T.H. Walther, A.S. Ulrich.
Length matters: functional flip of the short TatA transmembrane helix. Biophys. J. 122, 2125-2146 (2023)
DOI: 10.1016/j.bpj.2022.12.016
E. Strandberg, P. Wadhwani, J. Bürck, P. Anders, C. Mink, J. van den Berg, R.A.M. Ciriello, M.N. Melo, M.A.R.B. Castanho, E. Bardají, J.P. Ulmschneider, A.S. Ulrich. Temperature‐Dependent Re‐alignment of the Short Multifunctional Peptide BP100 in Membranes Revealed by Solid‐State NMR Spectroscopy and Molecular Dynamics Simulations. ChemBioChem 24, e202200602 (2022)
DOI: 10.1002/cbic.202200602
K.M. Thushara, M.R. Ponraj, S. Mandal, X. Peng, S.L. Grage, R. Venkatesan, T. Velmurugan, A.M. Kannan, V.K. Pillai, J.W. Kang, J. Bhagavathsingh.
Interlayer, gallery-engineered graphene oxide using selective protection of Mono-Boc-ethylenediamine as anode for sodium ion batteries. J. Energy Storage 73, 109237 (2023)
DOI: 10.1016/j.est.2023.109237
B. Zerulla, C. Li, D. Beutel, S. Oßwald, C. Holzer, J. Bürck, S. Bräse, C. Wöll, I. Fernandez-Corbaton, L. Heinke, C. Rockstuhl, M. Krstić. Exploring functional photonic devices made from a chiral metal–organic framework material by a multiscale computational method. Adv. Funct. Mater. 2301093 (2023)
DOI: 10.1002/adfm.202301093
Publications 2022
V.E. Diyuk, A.N. Zaderko, L.M. Grishchenko, S. Afonin, R. Mariychuk, M. Kaňuchová, V.V. Lisnyak. Preparation, texture and surface chemistry characterization of nanoporous-activated carbons co-doped with fluorine and chlorine. Applied Nanoscience 12, 2103–2116 (2022)
DOI:10.1007/s13204-022-02459-w
 V.E. Diyuk, A.N. Zaderko, L.M. Grishchenko, S. Afonin, R. Mariychuk, O.Y. Boldyrieva, V.A. Skryshevsky, M. Kaňuchová, V.V. Lisnyak. Surface chemistry of fluoroalkylated nanoporous activated carbons: XPS and 19F NMR study.. Applied Nanoscience 12, 637-650 (2022)
V.E. Diyuk, A.N. Zaderko, L.M. Grishchenko, S. Afonin, R. Mariychuk, O.Y. Boldyrieva, V.A. Skryshevsky, M. Kaňuchová, V.V. Lisnyak. Surface chemistry of fluoroalkylated nanoporous activated carbons: XPS and 19F NMR study.. Applied Nanoscience 12, 637-650 (2022)
DOI: 10.1007/s13204-021-01717-7
 S.L. Grage, S. Afonin, M. Ieronimo, M. Berditsch, P. Wadhwani, A.S. Ulrich. Probing and manipulating the lateral pressure profile in lipid bilayers using membrane-active peptides - A solid-state 19F NMR study. Intl. J. Mol. Sci. 23, 4544 (2022)
S.L. Grage, S. Afonin, M. Ieronimo, M. Berditsch, P. Wadhwani, A.S. Ulrich. Probing and manipulating the lateral pressure profile in lipid bilayers using membrane-active peptides - A solid-state 19F NMR study. Intl. J. Mol. Sci. 23, 4544 (2022)
DOI:10.3390/ijms23094544
J.L.S. Lopes, C.C.F. Araujo, R.C. Neves, J. Bürck, S.G. Couto. Structural analysis of the peptides temporin-Ra and temporin-Rb and interactions with model membranes. Eur. Biophys. J. 51, 493–502 (2022)
DOI:10.1007/s00249-022-01615-y
I.V. Komarov, G. Tolstanova, H. Kuznietsova, N. Dziubenko, P.I. Yanchuk, L.Y. Shtanova, S.P. Veselsky, L.V. Garmanchuk, N. Khranovska, O. Gorbach, T. Dovbynchuk, P. Borysko, O. Babii, T. Schober, A.S. Ulrich, S. Afonin. Towards in vivo photomediated delivery of anticancer peptides: Insights from pharmacokinetic and -dynamic data. J. Photochem. Photobiol. B 233, 112479 (2022)
DOI:10.1016/j.jphotobiol.2022.112479
S. Kröll, L. Schneider, P. Wadhwani, K.S. Rabe, C.M. Niemeyer. Orthogonal protein decoration of DNA nanostructures based on SpyCatcher–SpyTag interaction. Chem. Commun. 58, 13471 (2022)
DOI: 10.1039/d2cc05335g
 B. Meng, S.L. Grage, O. Babii, M. Takamiya, N. MacKinnon, T. Schober, I. Hutskalov, O. Nassar, S. Afonin, S. Koniev, I.V. Komarov, J.G. Korvink, U. Strähle, A.S. Ulrich. Highly fluorinated peptide probes with enhanced in vivo stability for 19F‐MRI. Small 18, 2107308 (2022)
B. Meng, S.L. Grage, O. Babii, M. Takamiya, N. MacKinnon, T. Schober, I. Hutskalov, O. Nassar, S. Afonin, S. Koniev, I.V. Komarov, J.G. Korvink, U. Strähle, A.S. Ulrich. Highly fluorinated peptide probes with enhanced in vivo stability for 19F‐MRI. Small 18, 2107308 (2022)
DOI:10.1002/smll.202107308
F. Schweigardt, E. Strandberg, P. Wadhwani, J. Reichert, J. Bürck, H.L.P. Cravo, L. Burger, A.S. Ulrich. Membranolytic mechanism of amphiphilic antimicrobial β-stranded [KL]n peptides. Biomedicines 10, 2071 (2022)
DOI: 10.3390/biomedicines10092071
Publications 2021
S. Afonin, S. Koniev, L. Préau, M. Takamiya, A.V. Strizhak, O. Babii, A. Hrebonkin, V.G. Pivovarenko, M. Dathe, F. le Noble, S. Rastegar, U. Strähle, A. S. Ulrich, I. V. Komarov. In vivo behavior of the antibacterial peptide cyclo[RRRWFW], explored using a 3-hydroxychromone-derived fluorescent amino acid. Frontiers Chem. 688446 (2021)
DOI: 10.3389/fchem.2021.688446
O. Babii, S. Afonin, C. Diel, M. Huhn, J. Dommermuth, T. Schober, S. Koniev, A. Hrebonkin, A. Nesterov-Mueller, I.V. Komarov, A.S. Ulrich. Diarylethene-Based Photoswitchable Inhibitors of Serine Proteases. Angew. Chem. 60, 21789-21794.
DOI: 10.1002/anie.202108847
 S.L. Grage, A. Culetto, A.S. Ulrich, S. Weinschenk. Membrane-mediated activity of local anesthetics. Mol. Pharmacol. 100, 502-512 (2021)
S.L. Grage, A. Culetto, A.S. Ulrich, S. Weinschenk. Membrane-mediated activity of local anesthetics. Mol. Pharmacol. 100, 502-512 (2021)
DOI: 10.1124/molpharm.121.000252
 A. B. Kanj, J. Bürck, N. Vankova, C. Li, D. Mutruc, A. Chandresh,S. Hecht, T. Heine, L. Heinke. Chirality remote control in nanoporous materials by circularly polarized light. J. Am. Chem. Soc. 143, 7059−7068 (2021)
A. B. Kanj, J. Bürck, N. Vankova, C. Li, D. Mutruc, A. Chandresh,S. Hecht, T. Heine, L. Heinke. Chirality remote control in nanoporous materials by circularly polarized light. J. Am. Chem. Soc. 143, 7059−7068 (2021)
DOI: 10.1021/jacs.1c01693
 N. Kassem, R. Araya-Secchi, K. Bugge, A. Barclay, H. Steinocher, A. Khondker, Y. Wang, A.J. Lenard, J. Bürck, C. Sahin, A.S. Ulrich, M. Landreh, M.C. Pedersen, M.C. Rheinstädter, P.A. Pedersen, K. Lindorff-Larsen, L. Arleth, B.B. Kragelund. Order and disorder - An integrative structure of the full-length human growth hormone receptor. Sci. Adv. 7, eabh3805 (2021)
N. Kassem, R. Araya-Secchi, K. Bugge, A. Barclay, H. Steinocher, A. Khondker, Y. Wang, A.J. Lenard, J. Bürck, C. Sahin, A.S. Ulrich, M. Landreh, M.C. Pedersen, M.C. Rheinstädter, P.A. Pedersen, K. Lindorff-Larsen, L. Arleth, B.B. Kragelund. Order and disorder - An integrative structure of the full-length human growth hormone receptor. Sci. Adv. 7, eabh3805 (2021)
DOI: 10.1126/sciadv.abh3805
I. V. Komarov, S. Afonin, O. Babii, T. Schober, A. S. Ulrich, Diarylethenes – molecules with good memory. in: Molecular Photoswitches – Synthesis, Properties and Applications (Editor Z. Pianowski), Wiley-VCH. in press (2021)
 V. Kubyshkin, J. Bürck, O. Babii, N. Budisa, A.S. Ulrich. Remarkably high solvatochromism in the circular dichroism spectra of the polyproline-II conformation: limitations or new opportunities? Phys. Chem. Chem. Phys. 23, 26931-26939 (2021)
V. Kubyshkin, J. Bürck, O. Babii, N. Budisa, A.S. Ulrich. Remarkably high solvatochromism in the circular dichroism spectra of the polyproline-II conformation: limitations or new opportunities? Phys. Chem. Chem. Phys. 23, 26931-26939 (2021)
DOI: 10.1039/d1cp04551b
 C. Mink, E. Strandberg, P. Wadhwani, M.N. Melo, J. Reichert, I. Wacker, M.A.R.B. Castanho, A.S. Ulrich. Overlapping properties of the short membrane-active peptide BP100 with (i) polycationic TAT and (ii) α-helical magainin family peptides. Front. Cell. Infect. Microbiol. 11, 609542 (2021)
C. Mink, E. Strandberg, P. Wadhwani, M.N. Melo, J. Reichert, I. Wacker, M.A.R.B. Castanho, A.S. Ulrich. Overlapping properties of the short membrane-active peptide BP100 with (i) polycationic TAT and (ii) α-helical magainin family peptides. Front. Cell. Infect. Microbiol. 11, 609542 (2021)
Doi: 10.3389/fcimb.2021.609542
 S. Nie, K.-F. Ratzsch, S.L. Grage, J. Keller, A.S. Ulrich, J. Lacayo-Pineda, M. Wilhelm. Correlation between macroscopic elasticity and chain dynamics of natural rubber during vulcanization as determined by a unique rheo-NMR combination. Macromol. 54, 6090-6100 (2021)
S. Nie, K.-F. Ratzsch, S.L. Grage, J. Keller, A.S. Ulrich, J. Lacayo-Pineda, M. Wilhelm. Correlation between macroscopic elasticity and chain dynamics of natural rubber during vulcanization as determined by a unique rheo-NMR combination. Macromol. 54, 6090-6100 (2021)
DOI: 10.1021/acs.macromol.1c00354
 K. R. Sahoo, R. Sharma, S. Bawari, Vivek S., P. K. Rastogi, S.S. Nair, S.L. Grage, T.N. Narayanan. Room-temperature ferromagnetic wide bandgap semiconducting fluorinated graphene-hBN vertical heterostructures. Materials Today Physics 21, 100547 (2021)
K. R. Sahoo, R. Sharma, S. Bawari, Vivek S., P. K. Rastogi, S.S. Nair, S.L. Grage, T.N. Narayanan. Room-temperature ferromagnetic wide bandgap semiconducting fluorinated graphene-hBN vertical heterostructures. Materials Today Physics 21, 100547 (2021)
DOI: 10.1016/j.mtphys.2021.100547
 Y. O. Shaydyuk, N. V. Bashmakova, A. M. Dmytruk, O. D. Kachkovsky, S. Koniev, A. V. Strizhak, I. V. Komarov, K. D. Belfield, M. V. Bondar, O. Babii. Nature of fast relaxation processes and spectroscopy of a membrane-active peptide modified with fluorescent amino acid exhibiting excited state intramolecular proton transfer and efficient stimulated emission. ACS Omega 6, 10119-10128 (2021)
Y. O. Shaydyuk, N. V. Bashmakova, A. M. Dmytruk, O. D. Kachkovsky, S. Koniev, A. V. Strizhak, I. V. Komarov, K. D. Belfield, M. V. Bondar, O. Babii. Nature of fast relaxation processes and spectroscopy of a membrane-active peptide modified with fluorescent amino acid exhibiting excited state intramolecular proton transfer and efficient stimulated emission. ACS Omega 6, 10119-10128 (2021)
DOI: 10.1021/acsomega.1c00193
E. S trandberg, P. Wadhwani, A.S. Ulrich. Antibiotic potential and biophysical characterization of amphipathic β-stranded [XZ]n peptides with alternating cationic and hydrophobic residues. Front. Med. Technol. 3, 622096 (2021)
trandberg, P. Wadhwani, A.S. Ulrich. Antibiotic potential and biophysical characterization of amphipathic β-stranded [XZ]n peptides with alternating cationic and hydrophobic residues. Front. Med. Technol. 3, 622096 (2021)
Doi: 10.3389/fmedt.2021.622096
 P. Sun, T. Scharnweber, P. Wadhwani, K.S. Rabe, and C.M. Niemeyer. DNA-directed assembly of a cell-responsive biohybrid interface for cargo release. Small Methods 2001049 (2021)
P. Sun, T. Scharnweber, P. Wadhwani, K.S. Rabe, and C.M. Niemeyer. DNA-directed assembly of a cell-responsive biohybrid interface for cargo release. Small Methods 2001049 (2021)
DOI: 10.1002/smtd.202001049
 N. Suryadevara, A. Pausch, E. Moreno-Pineda, A. Mizuno, J. Bürck, A. Baksi, T. Hochdörffer, I. Šalitroš, A.S. Ulrich, M.M. Kappes, V. Schünemann, W. Klopper, M. Ruben. Chiral Resolution of Spin-Crossover Active Iron(II) [2x2] Grid Complexes. Chemistry Eur. J. 27, 15172-15180 (2021)
N. Suryadevara, A. Pausch, E. Moreno-Pineda, A. Mizuno, J. Bürck, A. Baksi, T. Hochdörffer, I. Šalitroš, A.S. Ulrich, M.M. Kappes, V. Schünemann, W. Klopper, M. Ruben. Chiral Resolution of Spin-Crossover Active Iron(II) [2x2] Grid Complexes. Chemistry Eur. J. 27, 15172-15180 (2021)
DOI: 10.1002/chem.202101432
![]() P. Wadhwani, S. Sekaran, E. Strandberg, J. Bürck, A. Chugh, A.S. Ulrich. Membrane interactions of latarcins: antimicrobial peptides from spider venom. Int. J. Mol. Sci. 22, 10156 (2021)
P. Wadhwani, S. Sekaran, E. Strandberg, J. Bürck, A. Chugh, A.S. Ulrich. Membrane interactions of latarcins: antimicrobial peptides from spider venom. Int. J. Mol. Sci. 22, 10156 (2021)
DOI: 10.3390/ijms221810156.
 Z. Wang, H. Cui, M. Liu, S.L. Grage, M. Hoffmann, E. Sedghamiz, M. Wilhelm, W. Wenzel, P.A. Levkin. Tough, transparent, 3D printable and self-healing polyethylene glycol-gel (PEGgel). Adv. Materials (2021) in press
Z. Wang, H. Cui, M. Liu, S.L. Grage, M. Hoffmann, E. Sedghamiz, M. Wilhelm, W. Wenzel, P.A. Levkin. Tough, transparent, 3D printable and self-healing polyethylene glycol-gel (PEGgel). Adv. Materials (2021) in press
Publications 2020
 S. Afonin, O. Babii, A. Reuter, V. Middel, M. Takamiya, U. Strähle, I.V. Komarov, A.S. Ulrich. Light-controllable dithienylethene-modified cyclic peptides: photoswitching the in vivo toxicity in zebrafish embryos. Beilstein J. Org. Chem. 16, 38-49 (2020)
S. Afonin, O. Babii, A. Reuter, V. Middel, M. Takamiya, U. Strähle, I.V. Komarov, A.S. Ulrich. Light-controllable dithienylethene-modified cyclic peptides: photoswitching the in vivo toxicity in zebrafish embryos. Beilstein J. Org. Chem. 16, 38-49 (2020)
DOI: 10.3762/bjoc.16.6
 O. Babii, S. Afonin, T. Schober, L. V. Garmanchuk, L. I. Ostapchenko, V. Yurchenko, S. Zozulya, O. Tarasov, I. Pishel, A. S. Ulrich, I. V. Komarov. Peptide drugs for photopharmacology: how much of a safety advantage can be gained by photocontrol? Future Drug Discov. 2, FDD28 (2020)
O. Babii, S. Afonin, T. Schober, L. V. Garmanchuk, L. I. Ostapchenko, V. Yurchenko, S. Zozulya, O. Tarasov, I. Pishel, A. S. Ulrich, I. V. Komarov. Peptide drugs for photopharmacology: how much of a safety advantage can be gained by photocontrol? Future Drug Discov. 2, FDD28 (2020)
DOI: 10.4155/fdd-2019-0033
M. Brehm, S. Heissler, S. Afonin, P.A. Levkin. Nanomolar synthesis in droplet microarrays with UV-triggered on-chip cell screening. Small 1905971 (2020)
DOI: 10.1002/smll.201905971
M. Drouin, P. Wadhwani, S.L. Grage, J. Bürck, J. Reichert, S. Tremblay, M.S. Mayer, C. Diel, A. Staub, J.-F. Paquin, A.S. Ulrich. Monofluoroalkene-isostere as a 19F-NMR label for the peptide backbone: synthesis and evaluation in membrane-bound PGLa and (KIGAKI)3. Chemistry Eur. J. 26, 1511-1517 (2020)
DOI: 10.1002/chem.201905054

S. L. Grage, S. Afonin, A. S. Ulrich. 19F NMR in biomembranes. In "Solid state NMR: Applications in Biomembrane Structure" (Ed. F. Separovic and M. A. Sani), IOP Publishing, pages 6.1-6.43 (2020)
 W.-H. Hsu, T. Masuda, S. Afonin, T. Sakai, J.V.V. Arafiles, K. Kawano, H. Hirose, M. Imanishi, A.S.Ulrich, S. Futaki. Enhancing the activity of membrane remodeling epsin-peptide by trimerization. Bioorg. Med. Chem. Lett. 30, 127190 (2020)
W.-H. Hsu, T. Masuda, S. Afonin, T. Sakai, J.V.V. Arafiles, K. Kawano, H. Hirose, M. Imanishi, A.S.Ulrich, S. Futaki. Enhancing the activity of membrane remodeling epsin-peptide by trimerization. Bioorg. Med. Chem. Lett. 30, 127190 (2020)
DOI: 10.1016/j.bmcl.2020.127190
 M. Serulla, G. Ichim, F. Stojceski, G. Grasso, S. Afonin, M. Heulot, T. Schober, R. Roth, C. Godefroy, P.-E. Milhiet, K. Das, A. J. García-Sáez, A. Danani, C. Widmann. TAT-RasGAP317-326 kills cells by targeting inner-leaflet–enriched phospholipids. Proc. Natl. Acad. Sci. USA 117, 31871-31881 (2020)
M. Serulla, G. Ichim, F. Stojceski, G. Grasso, S. Afonin, M. Heulot, T. Schober, R. Roth, C. Godefroy, P.-E. Milhiet, K. Das, A. J. García-Sáez, A. Danani, C. Widmann. TAT-RasGAP317-326 kills cells by targeting inner-leaflet–enriched phospholipids. Proc. Natl. Acad. Sci. USA 117, 31871-31881 (2020)
DOI: 10.1073/pnas.2014108117
R. Sharma, T. C. Asmara, K. Rani Sahoo, S. L. Grage, R. Zhang, J. Sun, S. Das, A. S. Ulrich, A. Rusydi, S. Aryasomayajula, R. Paulmurugan, D. Liepmann, D. Sakthi Kumar, P. Somasundaran, V. Renugopalakrishnan, T. N. Narayanan. Structural and Electronic Transport Properties of Fluorographene Directly Grown on Silicates for Possible Biosensor Applications. ACS Appl. Nano Mater. 3, 5399–5409 (2020)
DOI: 10.1021/acsanm.0c00768
 L. M. E. Steger, A. Kohlmeyer, P. Wadhwani, J. Bürck, E. Strandberg, J. Reichert, S. L. Grage, S. Afonin, M. Kempfer, A. C. Görner, J. Koch, T. H. Walther, A. S. Ulrich. Structural and functional characterization of the pore forming domain of pinholin S2168. Proc. Natl. Acad. Sci. 117, 29637-29646 (2020)
L. M. E. Steger, A. Kohlmeyer, P. Wadhwani, J. Bürck, E. Strandberg, J. Reichert, S. L. Grage, S. Afonin, M. Kempfer, A. C. Görner, J. Koch, T. H. Walther, A. S. Ulrich. Structural and functional characterization of the pore forming domain of pinholin S2168. Proc. Natl. Acad. Sci. 117, 29637-29646 (2020)
DOI: 10.1073/pnas.2007979117
 E. Strandberg, F. Schweigardt, P. Wadhwani, J. Bürck, J. Reichert, H. L. P. Cravo, L. Burger, A. S. Ulrich. Phosphate‑dependent aggregation of [KL]n peptides affects their membranolytic activity. Sci. Rep. 10, 12300 (2020)
E. Strandberg, F. Schweigardt, P. Wadhwani, J. Bürck, J. Reichert, H. L. P. Cravo, L. Burger, A. S. Ulrich. Phosphate‑dependent aggregation of [KL]n peptides affects their membranolytic activity. Sci. Rep. 10, 12300 (2020)
DOI: 10.1038/s41598-020-69162-0
 E. Strandberg, D. Bentz, P. Wadhwani, A. S. Ulrich. Chiral supramolecular architecture of stable transmembrane pores formed by an α-helical antibiotic peptide in the presence of lyso-lipids. Sci. Rep. 10, 4710 (2020)
E. Strandberg, D. Bentz, P. Wadhwani, A. S. Ulrich. Chiral supramolecular architecture of stable transmembrane pores formed by an α-helical antibiotic peptide in the presence of lyso-lipids. Sci. Rep. 10, 4710 (2020)
DOI: 10.1038/s41598-020-61526-w
 E. Strandberg, D. Bentz, P. Wadhwani, J. Bürck, A. S. Ulrich. Terminal charges modulate the pore forming activity of cationic amphipathic helices. Biochim. Biophys. Acta 1862, 183243 (2020)
E. Strandberg, D. Bentz, P. Wadhwani, J. Bürck, A. S. Ulrich. Terminal charges modulate the pore forming activity of cationic amphipathic helices. Biochim. Biophys. Acta 1862, 183243 (2020)
DOI: 10.1016/j.bbamem.2020.183243

 A. V. Strizhak, O. Babii, S. Afonin, I. Bakanovich, T. Pantelejevs, W. Xu, E. Fowler, R. Eapen, K. Sharma, M. O. Platonov, V. V. Hurmach, L. Itzhaki, M. Hyvönen, A. S. Ulrich, D. R. Spring, I. V. Komarov. Diarylethene moiety as an enthalpy-entropy switch: photoisomerizable stapled peptides for modulating p53/MDM2 interaction. Org. Biomol. Chem. 18, 5359-5369 (2020)
A. V. Strizhak, O. Babii, S. Afonin, I. Bakanovich, T. Pantelejevs, W. Xu, E. Fowler, R. Eapen, K. Sharma, M. O. Platonov, V. V. Hurmach, L. Itzhaki, M. Hyvönen, A. S. Ulrich, D. R. Spring, I. V. Komarov. Diarylethene moiety as an enthalpy-entropy switch: photoisomerizable stapled peptides for modulating p53/MDM2 interaction. Org. Biomol. Chem. 18, 5359-5369 (2020)
DOI: 10.1039/d0ob00831a
S. Wörner, F. Rönicke, A.S. Ulrich, H.A. Wagenknecht. 4-Aminophthalimide as small and environment-sensitive fluorescent amino acid to probe transmembrane peptides. ChemBioChem 21, 618-622 (2020)
DOI: 10.1002/cbic.201900520
Z. You, M. Behl, S.L. Grage, J. Bürck, Q. Zhao, A.S. Ulrich, A. Lendlein. Shape-memory effect by sequential coupling of functions over different length scales in an architectured hydrogel. Biomacromolecules 21, 680-687 (2020)
DOI: 10.1021/acs.biomac.9b01390
Publications 2019
 M. Berditsch, S. Afonin, J. Reuster, H. Lux, K. Schkolin, O. Babii, D.S. Radchenko, I. Abdullah, N. William, V. Middel, U. Strähle, A. Nelson, K. Valko, A.S. Ulrich. Supreme activity of gramicidin S against resistant, persistent and biofilm cells of staphylococci and enterococci. Sci. Rep. 9, 17983 (2019)
M. Berditsch, S. Afonin, J. Reuster, H. Lux, K. Schkolin, O. Babii, D.S. Radchenko, I. Abdullah, N. William, V. Middel, U. Strähle, A. Nelson, K. Valko, A.S. Ulrich. Supreme activity of gramicidin S against resistant, persistent and biofilm cells of staphylococci and enterococci. Sci. Rep. 9, 17983 (2019)
DOI: 10.1038/s41598-019-54212-z
 B. Casciaro, Q. Lin, Q., S. Afonin, M.R. Loffredo, V. de Turris, V. Middel, A.S. Ulrich, Y.P. Di, L.M. Mangoni. Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a(1-21)NH2. FEBS Journal 286, 3874-3891 (2019)
B. Casciaro, Q. Lin, Q., S. Afonin, M.R. Loffredo, V. de Turris, V. Middel, A.S. Ulrich, Y.P. Di, L.M. Mangoni. Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a(1-21)NH2. FEBS Journal 286, 3874-3891 (2019)
DOI: 10.1111/febs.14940
 I.‐L. Hsiao, S. Fritsch‐Decker, A. Leidner, M. Al‐Rawi, V. Hug, S. Diabaté, S.L. Grage, M. Meffert, T. Stoeger, D. Gerthsen, A.S. Ulrich, C.M. Niemeyer, C. Weiss. Biocompatibility of amine‐functionalized silica nanoparticles: The role of surface coverage. Small 1805400 (2019)
I.‐L. Hsiao, S. Fritsch‐Decker, A. Leidner, M. Al‐Rawi, V. Hug, S. Diabaté, S.L. Grage, M. Meffert, T. Stoeger, D. Gerthsen, A.S. Ulrich, C.M. Niemeyer, C. Weiss. Biocompatibility of amine‐functionalized silica nanoparticles: The role of surface coverage. Small 1805400 (2019)
DOI: 10.1002/smll.201805400
 A.B. Kanj, J. Bürck, S. Grosjean, S. Bräse, L. Heinke. Switching the enantioselectivity of nanoporous host materials by light. Chem. Commun. 55, 8776 (2019)
A.B. Kanj, J. Bürck, S. Grosjean, S. Bräse, L. Heinke. Switching the enantioselectivity of nanoporous host materials by light. Chem. Commun. 55, 8776 (2019)
DOI: 10.1039/C9CC02849H
![]() I.V. Komarov, S. Afonin, A.S. Ulrich. 19F-labelled amino acids for NMR structure analysis of membrane-bound peptides, in "Fluorine in life sciences: pharmaceuticals, medicinal diagnostics, and agrochemicals" (Eds. G. Haufe and F. Léroux)
I.V. Komarov, S. Afonin, A.S. Ulrich. 19F-labelled amino acids for NMR structure analysis of membrane-bound peptides, in "Fluorine in life sciences: pharmaceuticals, medicinal diagnostics, and agrochemicals" (Eds. G. Haufe and F. Léroux)
DOI: 10.1016/B978-0-12-812733-9.00010-6
 V. Kubyshkin, S.L. Grage, A. S. Ulrich, N. Budisa. Bilayer thickness determines the alignment of model
V. Kubyshkin, S.L. Grage, A. S. Ulrich, N. Budisa. Bilayer thickness determines the alignment of model
polyproline helices in lipid membranes. Phys. Chem. Chem. Phys. 21, 22396-22408 (2019)
DOI: 10.1039/c9cp02996f
 P.S. Kumagai, V.K. Sousa, M. Donato, R. Itri, L.M. Beltramini, A.P.U. Araujo, J. Bürck, B.A. Wallace, J.L.S. Lopes. Unveiling the binding and orientation of the antimicrobial peptide Plantaricin 149 in zwitterionic and negatively charged membranes. Eur. Biophys. J. 48, 621–633 (2019)
P.S. Kumagai, V.K. Sousa, M. Donato, R. Itri, L.M. Beltramini, A.P.U. Araujo, J. Bürck, B.A. Wallace, J.L.S. Lopes. Unveiling the binding and orientation of the antimicrobial peptide Plantaricin 149 in zwitterionic and negatively charged membranes. Eur. Biophys. J. 48, 621–633 (2019)
DOI: 10.1007/s00249-019-01387-y
P.-A. Paquet-Côté, M. Fillion, M.-È. Provencher, F. Otis, J. Dionne, S. Cardinal, B. Collignon, J. Bürck, P. Lagüe, A.S. Ulrich, M. Auger, N. Voyer. Crown ether modified peptide interactions with model membranes. Supramol. Chem. 31, 159-171 (2019)
DOI: 10.1080/10610278.2019.1574349
A. Raasakka, S. Ruskamo, J. Kowal, H. Han, A. Baumann, M. Myllykoski, A. Fasano, R. Rossano, P. Riccio, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Molecular structure and function of myelin protein P0 in membrane stacking. Sci. Rep. 9:642 (2019)
DOI: 10.1038/s41598-018-37009-4
 V. Schneider, P. Wadhwani, J. Reichert, J. Bürck, M. Elstner, A.S. Ulrich, T. Kubar. Tetrameric charge-zipper assembly of the TisB peptide in membranes - computer simulation and experiment. J. Phys. Chem. B 123, 1770-1779 (2019)
V. Schneider, P. Wadhwani, J. Reichert, J. Bürck, M. Elstner, A.S. Ulrich, T. Kubar. Tetrameric charge-zipper assembly of the TisB peptide in membranes - computer simulation and experiment. J. Phys. Chem. B 123, 1770-1779 (2019)
DOI: 10.1021/acs.jpcb.8b12087
 T. Schober, I. Wehl, S. Afonin, O. Babii, A. Iampolska, U. Schepers, I.V. Komarov, A.S. Ulrich. Controlling the uptake of diarylethene‐based cell‐penetrating peptides into cells using light. ChemPhotoChem 3, 384-391 (2019)
T. Schober, I. Wehl, S. Afonin, O. Babii, A. Iampolska, U. Schepers, I.V. Komarov, A.S. Ulrich. Controlling the uptake of diarylethene‐based cell‐penetrating peptides into cells using light. ChemPhotoChem 3, 384-391 (2019)
DOI: 10.1002/cptc.201900019
 C. Schweigert, O. Babii, S. Afonin, T. Schober, N.C. Michenfelder, J. Leier, I.V. Komarov, A.S. Ulrich, and A.N. Unterreiner. Real-time observation of dithienylethene-based photoswitches in a cyclic peptide environment. ChemPhotoChem 3, 403-410 (2019)
C. Schweigert, O. Babii, S. Afonin, T. Schober, N.C. Michenfelder, J. Leier, I.V. Komarov, A.S. Ulrich, and A.N. Unterreiner. Real-time observation of dithienylethene-based photoswitches in a cyclic peptide environment. ChemPhotoChem 3, 403-410 (2019)
DOI: 10.1002/cptc.201900005
E. Strandberg, A.S. Ulrich. Flow charts for the systematic solid-state 19F/2H-NMR structure analysis of membrane-bound peptides. Ann. Rep. NMR Spect. 99, 79 (2019)
DOI: 0.1016/bs.arnmr.2019.08.002
M. Tabasinezhad, F. Mahboudi, W. Wenzel, H. Rahimi, T. Walther, C. Blattner, E. Omidinia. The transient production of anti-TNF-α antibody Adalimumab and a comparison of its characterization to the biosimilar Cinorra. Prot. Expr. Purif. 155, 59-65 (2019)
DOI: 10.1016/j.pep.2018.11.006
 A.N. Zaderko, R.Y. Shvets, I.I. Grygorchak, S. Afonin, V.E. Diyuk, R.T. Mariychuk, O.Y. Boldyrieva, M. Kaňuchová, V.V. Lisnyak. Fluoroalkylated nanoporous carbons: Testing as a supercapacitor electrode. Appl. Surf. Sci. 470, 882-892 (2019)
A.N. Zaderko, R.Y. Shvets, I.I. Grygorchak, S. Afonin, V.E. Diyuk, R.T. Mariychuk, O.Y. Boldyrieva, M. Kaňuchová, V.V. Lisnyak. Fluoroalkylated nanoporous carbons: Testing as a supercapacitor electrode. Appl. Surf. Sci. 470, 882-892 (2019)
DOI: 10.1016/j.apsusc.2018.11.141
Publications 2018
V. Kubyshkin, S.L. Grage, J. Bürck, A.S. Ulrich, N. Budisa. Transmembrane polyproline helix. J. Phys. Chem. Lett. 9, 2170–2174 (2018)
DOI :10.1021/acs.jpclett.8b00829
O. Babii, S. Afonin, A.Y. Ishchenko, T. Schober, A.O. Negelia, G.M. Tolstanova, L.V. Garmanchuk, L.I. Ostapchenko, I.V. Komarov, A.S. Ulrich. Structure-activity relationships of photoswitchable diarylethene-based β-hairpin peptides as membranolytic antimicrobial and anticancer agents. J. Med. Chem. 61, 10793-10813 (2018)
DOI: 10.1021/acs.jmedchem.8b01428
S. Reißer, E. Strandberg, T. Steinbrecher, M. Elstner, A.S. Ulrich. Best of two worlds? How MD simulations of amphiphilic helical peptides in membranes can complement data from oriented solid-state NMR. J. Chem. Theory Comput. 14, 6002-6014 (2018)
DOI: 10.1021/acs.jctc.8b00283
S.L. Grage, S. Kara, A. Bordessa, V. Doan, F. Rizzolo, M. Putzu, T. Kubař, A.M. Papini, G. Chaume, T. Brigaud, S. Afonin, A.S. Ulrich. Orthogonal 19F-labeling for solid-state NMR spectroscopy reveals the conformation and orientation of short peptaibols in membranes. Chemistry Eur. J. 24, 4328–4335 (2018)
DOI: 10.1002/chem.201704307
I.V. Komarov, S. Afonin, O. Babii, T. Schober, A.S. Ulrich. Efficiently photocontrollable or not? Biological activity of photoisomerizable diarylethenes. Chemistry Eur. J. 24, 11245-11254 (2018)
DOI: 10.1002/chem.201801205
H. Zamora-Carreras, B. Maestro, E. Strandberg, A.S. Ulrich, J.M. Sanz, M.Á. Jiménez. Roles of amphipathicity and hydrophobicity in the micelle-driven structural switch of a 14-mer peptide core from a choline-binding repeat. Chemistry Eur. J. 24, 5825–5839 (2018)
DOI: 10.1002/chem.201704802
E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Bürck, F. Rabanal, A.S. Ulrich. Helix fraying and lipid-dependent structure of a short amphipathic membrane-bound peptide revealed by solid-state NMR. J. Phys. Chem. B 122, 6236-6250 (2018)
DOI: 10.1021/acs.jpcb.8b02661
P. Ridone, S.L. Grage, A. Patkunarajah, A.R. Battle, A.S. Ulrich, B. Martinac. "Force-from-lipids" gating of mechanosensitive channels modulated by PUFAs. J. Mech. Beh. Biomed. Mat. 79, 158-167 (2018)
DOI: 10.1016/j.jmbbm.2017.12.026
M.-C. Gagnon, E. Strandberg, A.S. Ulrich, J.-F. Paquin, M. Auger. New insights into the influence of monofluorination on dimyristoylphosphatidylcholine membrane properties: A solid-state NMR study. Biochim. Biophys. Acta - Biomembranes 1860, 654-663 (2018)
DOI: 10.1016/j.bbamem.2017.12.002
A.V. Strizhak, K. Sharma, O. Babii, S. Afonin, A.S. Ulrich, I.V. Komarov, D.R. Spring. Highly reactive bis-cyclooctyne-modified diarylethene for SPAAC-mediated cross-linking. Org. Biomol. Chem. 16, 8559–8564 (2018)
DOI: 10.1039/c8ob02428f
O.M. Michurin, K. Tolmachova, S. Afonin, O. Babii, S.L. Grage, A.S. Ulrich, I.V. Komarov, D.S. Radchenko. Conformationally constrained mono-fluorinated arginine as a cationic label for solid-state 19F NMR analysis of membrane-bound peptides. Eur. J. Org. Chem. 2018, 3826–3833 (2018)
DOI: 10.1002/ejoc.201800473
J. Bürck, O. Aras, L. Bertinetti, C.A. Ilhan, M.A. Ermeydan, R. Schneider, A.S. Ulrich, M. Kazanci. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 1151, 73-80 (2018)
DOI: 10.1016/j.molstruc.2017.09.030
S. Reißer, S. Prock, H. Heinzmann, A.S. Ulrich. Protein ORIGAMI: A program for the creation of 3D paper models of folded peptides. Biochem. Mol. Biol. Education 46, 403–409 (2018)
DOI: 10.1002/bmb.21132
Publications 2017
S. Afonin, V. Kubyshkin, P.K. Mykhailiuk, I.V. Komarov, A.S. Ulrich. Conformational plasticity of the cell-penetrating peptide SAP as revealed by solid-state 19F-NMR and circular dichroism spectroscopies. J. Phys. Chem. B 121, 6479-6491 (2017)
DOI: 10.1021/acs.jpcb.7b02852
O. Babii, S. Afonin, T. Schober, I.V. Komarov, A.S. Ulrich. Flexibility vs rigidity of amphipathic peptide conjugates when interacting with lipid bilayers. Biochim. Biophys. Acta 1859, 2505-2515 (2017)
DOI:10.1016/j.bbamem.2017.09.021
M. Berditsch, M. Trapp, S. Afonin, C. Weber, J. Misiewicz, J. Turkson, A.S. Ulrich. Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens. Sci. Rep. 7, 44324 (2017)
DOI: 10.1038/srep44324
K. Eggenberger, P. Sanyal, S. Hundt, P. Wadhwani, A.S. Ulrich, P. Nick. Challenge integrity: The cell-penetrating peptide BP100 interferes with the auxin-actin oscillator. Plant Cell Physiol. 58, 71-85 (2017)
DOI: 10.1093/pcp/pcw161
M.-C. Gagnon, E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Reichert, J. Bürck, F. Rabanal, M. Auger, J.-F. Paquin, A.S. Ulrich. Influence of length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochem. 56, 1680−1695 (2017) DOI: 10.1021/acs.biochem.6b01071
S.L. Grage, M.-A. Sani, O. Cheneval, S. Troeira Henriques, C. Schalck, R. Heinzmann, J.S. Mylne, P.K. Mykhailiuk, S. Afonin, I.V. Komarov, F. Separovic, D.J. Craik, A.S. Ulrich. Orientation and location of the cyclotide kalata B1 in phospholipid bilayers revealed by solid-state NMR. Biophys. J. 112, 630-642 (2017)
DOI: 10.1016/j.bpj.2016.12.040
S. Kara, S. Afonin, O. Babii, A.N. Tkachenko, I.V. Komarov, A.S. Ulrich. Diphytanoyl lipids as model systems for studying membrane-active peptides. Biochim. Biophys. Acta 1859, 1828-1837 (2017)
DOI: 10.1016/j.bbamem.2017.06.003
M.J. Klein, S. Schmidt, P. Wadhwani, J. Bürck, J.Reichert, S. Afonin, M. Berditsch, T. Schober, R. Brock, M. Kansy, A.S. Ulrich. Lactam-stapled cell-penetrating peptides: Cell uptake and membrane binding properties. J. Med. Chem. 60, 8071-8082 (2017)
DOI: 10.1021/acs.jmedchem.7b00813
L. Li, M. Schmitt, A. Matzke-Ogi, P. Wadhwani, V. Orian-Rousseau, P.A. Levkin. CD44v6-peptide functionalized nanoparticles selectively bind to metastatic cancer cells. Adv. Sci. 4, 1600202 (2017)
DOI: 10.1002/advs.201600202
P. Mühlhäuser, P. Wadhwani, E. Strandberg, J. Bürck, A.S. Ulrich. Structure analysis of the membrane-bound DCD-derived peptide SSL-25 from human sweat. Biochim. Biophys. Acta 1859, 2308-2318 (2017)
DOI: 10.1016/j.bbamem.2017.09.004
T. Murayama, T. Masuda, S. Afonin, K. Kawano, T. Takatani-Nakase, H. Ida, Y. Takahashi, T. Fukuma, A.S. Ulrich, S. Futaki. Loosening of lipid packing promotes oligoarginine entry into cells. Angew. Chem. Int. Ed. 56, 7644-7647 (2017)
DOI: 10.1002/anie.201703578
C. Priem, A. Wuttke, M. Berditsch, A.S. Ulrich, A. Geyer. Scaling the amphiphilic character and antimicrobial activity of Gramicidin S by dihydroxylation or ketal formation. J. Org. Chem. in press (2017)
DOI: 10.1021/acs.joc.7b02177
M. Putzu, S. Kara, S. Afonin, S.L. Grage, A. Bordessa, G. Chaume, T. Brigaud , A.S. Ulrich, T. Kubař. Structural behavior of the peptaibol Harzianin HK VI in a DMPC bilayer: Insights from MD simulations. Biophys. J. 112, 2602-2614 (2017)
DOI: 10.1016/j.bpj.2017.05.019
S. Reißer, D. Poger, M. Stroet, A.E. Mark. Real Cost of Speed: The Effect of a Time-Saving Multiple-Time-Stepping Algorithm on the Accuracy of Molecular Dynamics Simulations. J. Chem. Theory Comput. 13, 2367-2372 (2017)
DOI: 10.1021/acs.jctc.7b00178
A. Raasakka, S. Ruskamo, J. Kowal, R. Barker, A. Baumann, A. Martel, J. Tuusa, M. Myllykoski, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Sci. Rep. 7: 4974 (2017)
DOI:10.1038/s41598-017-05364-3
E. Strandberg, A.S. Ulrich. Solid-state 19F-NMR analysis of peptides in oriented biomembranes. In: Modern Magnetic Resonance, pages 1-18. (Springer International Publishing, 2017, ed. Graham A. Webb)
DOI: 10.1007/978-3-319-28275-6_88-1
E. Strandberg, A.S. Ulrich (2017). Solid-state NMR for studying peptide structures and peptide-lipid interactions in membranes. In: Modern Magnetic Resonance, pages 1-13. (Springer International Publishing, 2017, ed. Graham A. Webb)
DOI: 10.1007/978-3-319-28275-6_114-1
H. Turgut, A.C. Schmidt, P. Wadhwani, A. Welle, R. Müller, G. Delaittre. The para-fluoro-thiol ligation in water. Polymer Chem. 8, 1288-1293 (2017)
DOI: 10.1039/C6PY02108E
A. Turshatov, D. Busko, N. Kiseleva, S.L. Grage, I.A. Howard, B.S. Richards. Room-temperature high-efficiency solid-state triplet-triplet annihilation up-conversion in amorphous poly(olefin sulfone)s. ACS Appl. Mat. Interf. 9, 8280-8286 (2017)
DOI:10.1021/acsami.6b12625
J. Zerweck, E. Strandberg, O. Kukharenko, J. Reichert, J. Bürck, P. Wadhwani, A.S. Ulrich. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep. 7, 13153 (2017)
DOI: 10.1038/s41598-017-12599-7
P. Wadhwani, N. Heidenreich, B. Podeyn, J. Bürck, A.S. Ulrich. Antibiotic gold: tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater. Sci. 5, 817-827 (2017)
DOI: 10.1039/C7BM00069C
R. Wallbrecher, T. Ackels, R.A. Olea, M.J. Klein, L. Caillon, J. Schiller, P.H. Bovée-Geurts, T.H. van Kuppevelt, A.S. Ulrich, M. Spehr, M.J.W. Adjobo-Hermans, R. Brock. Membrane permeation of arginine-rich cell-penetrating peptides independent of transmembrane potential as a function of lipid composition and membrane fluidity. J. Contr. Release 256, 68-78 (2017)
DOI: 10.1016/j.jconrel.2017.04.013
Publications 2016
 O. Babii, S. Afonin, L.V. Garmanchuk, V.V. Nikulina, T.V. Nikolaienko, O.V. Storozhuk, D.V. Shelest, O.I. Dasyukevich, O.I., L.I. Ostapchenko, V. Iurchenko, S. Zozulya, A.S. Ulrich, I.V. Komarov. Direct photocontrol of peptidomimetics: an alternative to oxygen-dependent photodynamic cancer therapy. Angewandte Chemie - International Edition 55, 5493-5496 (2016)
O. Babii, S. Afonin, L.V. Garmanchuk, V.V. Nikulina, T.V. Nikolaienko, O.V. Storozhuk, D.V. Shelest, O.I. Dasyukevich, O.I., L.I. Ostapchenko, V. Iurchenko, S. Zozulya, A.S. Ulrich, I.V. Komarov. Direct photocontrol of peptidomimetics: an alternative to oxygen-dependent photodynamic cancer therapy. Angewandte Chemie - International Edition 55, 5493-5496 (2016)
DOI: 10.1002/anie.201600506
G. Batoni, M. Casu, A. Giuliani, V. Luca, G. Maisetta, M.L. Mangoni, G. Manzo, M. Pintus, G. Pirri, A.C. Rinaldi, M.A. Scorciapino, I. Serra, A.S. Ulrich, P. Wadhwani. Rational modification of a dendrimeric peptide with antimicrobial activity: Consequences on membrane-binding and biological properties. Amino Acids 48, 887-900 (2016)
DOI: 10.1007/s00726-015-2136-5
 M. Berditsch, H. Lux, O. Babii, S. Afonin, A.S. Ulrich. Therapeutic potential of gramicidin S in the treatment of root canal infections. Pharmaceuticals 9, 56 (2016)
M. Berditsch, H. Lux, O. Babii, S. Afonin, A.S. Ulrich. Therapeutic potential of gramicidin S in the treatment of root canal infections. Pharmaceuticals 9, 56 (2016)
DOI: 10.3390/ph9030056
 J. Bürck, P. Wadhwani, S. Fanghänel, A.S. Ulrich. Oriented Circular Dichroism: A Method to Characterize Membrane-Active Peptides in Oriented Lipid Bilayers. Acc. Chem. Res. 49, 184-192 (2016)
J. Bürck, P. Wadhwani, S. Fanghänel, A.S. Ulrich. Oriented Circular Dichroism: A Method to Characterize Membrane-Active Peptides in Oriented Lipid Bilayers. Acc. Chem. Res. 49, 184-192 (2016)
DOI: 10.1021/acs.accounts.5b00346
N. Gao, P. Wadhwani, P. Mühlhäuser, Q. Liu, M. Riemann, A.S. Ulrich, P. Nick. An antifungal protein from Ginkgo biloba binds actin and can trigger cell death. Protoplasma 253, 1159-1174 (2016)
DOI: 10.1007/s00709-015-0876-4
 S.L. Grage, S. Afonin, S. Kara, G. Buth, A.S. Ulrich. Membrane Thinning and Thickening Induced by Membrane-Active Amphipathic Peptides. Frontiers in Cell and Developmental Biology 4, 65 (2016)
S.L. Grage, S. Afonin, S. Kara, G. Buth, A.S. Ulrich. Membrane Thinning and Thickening Induced by Membrane-Active Amphipathic Peptides. Frontiers in Cell and Developmental Biology 4, 65 (2016)
DOI: 10.3389/fcell.2016.00065
 A. Grau-Campistany, E. Strandberg, P. Wadhwani, F. Rabanal, A.S. Ulrich. Extending the hydrophobic mismatch concept to amphiphilic membraneolytic peptides. J. Phys. Chem. Lett. 7, 1116-1120 (2016)
A. Grau-Campistany, E. Strandberg, P. Wadhwani, F. Rabanal, A.S. Ulrich. Extending the hydrophobic mismatch concept to amphiphilic membraneolytic peptides. J. Phys. Chem. Lett. 7, 1116-1120 (2016)
DOI: 10.1021/acs.jpclett.6b00136
 S.O. Kokhan, A.V. Tymtsunik, S.L. Grage, S. Afonin S., O. Babii, M. Berditsch, A.V. Strizhak, D. Bandak, M.O. Platonov, I.V. Komarov, A.S. Ulrich, P.K. Mykhailiuk. Design, synthesis, and application of an optimized monofluorinated aliphatic label for peptide studies by solid-state 19F NMR spectroscopy. Angewandte Chemie - Intl. Ed. 55, 14788-14792 (2016) DOI: 10.1002/anie.201608116
S.O. Kokhan, A.V. Tymtsunik, S.L. Grage, S. Afonin S., O. Babii, M. Berditsch, A.V. Strizhak, D. Bandak, M.O. Platonov, I.V. Komarov, A.S. Ulrich, P.K. Mykhailiuk. Design, synthesis, and application of an optimized monofluorinated aliphatic label for peptide studies by solid-state 19F NMR spectroscopy. Angewandte Chemie - Intl. Ed. 55, 14788-14792 (2016) DOI: 10.1002/anie.201608116
 O. M. Michurin, S. Afonin, M. Berditsch, C.G. Daniliuc, A.S. Ulrich, I.V. Komarov, D.S. Radchenko. Delivering structural information on the polar face of membrane-active peptides: 19F-NMR labels with a cationic side chain. Angewandte Chemie - International Edition 55, 14595-14599 (2016)
O. M. Michurin, S. Afonin, M. Berditsch, C.G. Daniliuc, A.S. Ulrich, I.V. Komarov, D.S. Radchenko. Delivering structural information on the polar face of membrane-active peptides: 19F-NMR labels with a cationic side chain. Angewandte Chemie - International Edition 55, 14595-14599 (2016)
DOI: 10.1002/anie.201607161
 D.S. Radchenko, S. Kattge, S. Kara, A.S. Ulrich A.S., S. Afonin. Does a methionine-to-norleucine substitution in PGLa influence peptide-membrane interactions? Biochim. Biophys. Acta 1858, 2019-27 (2016)
D.S. Radchenko, S. Kattge, S. Kara, A.S. Ulrich A.S., S. Afonin. Does a methionine-to-norleucine substitution in PGLa influence peptide-membrane interactions? Biochim. Biophys. Acta 1858, 2019-27 (2016)
DOI: 10.1016/j.bbamem.2016.06.002
![]() E. Strandberg, D. Horn, S. Reißer, J. Zerweck, P. Wadhwani, A.S. Ulrich. 2H-NMR and MD simulations reveal membrane-bound conformation of magainin 2 and its synergy with PGLa. Biophys. J. 111, 2149-2161 (2016)
E. Strandberg, D. Horn, S. Reißer, J. Zerweck, P. Wadhwani, A.S. Ulrich. 2H-NMR and MD simulations reveal membrane-bound conformation of magainin 2 and its synergy with PGLa. Biophys. J. 111, 2149-2161 (2016)
DOI: 10.1016/j.bpj.2016.10.012
 C.M. Thiele, A.S. Ulrich. Light flips a membrane-embedded helix. Science 352, 520 (2016)
C.M. Thiele, A.S. Ulrich. Light flips a membrane-embedded helix. Science 352, 520 (2016)
DOI: 10.1126/science.aaf6191
 H. Zamora-Carreras, E. Strandberg, P. Mühlhäuser, J. Bürck, P. Wadhwani, M. Ángeles Jiménez, M. Bruix, A.S. Ulrich. Alanine scan and 2H-NMR analysis of the membrane-active peptide BP100 points to a distinct carpet mechanism of action. Biochim. Biophys. Acta 1858, 1328-1338 (2016)
H. Zamora-Carreras, E. Strandberg, P. Mühlhäuser, J. Bürck, P. Wadhwani, M. Ángeles Jiménez, M. Bruix, A.S. Ulrich. Alanine scan and 2H-NMR analysis of the membrane-active peptide BP100 points to a distinct carpet mechanism of action. Biochim. Biophys. Acta 1858, 1328-1338 (2016)
DOI: 10.1016/j.bbamem.2016.03.014
 J. Zerweck, E. Strandberg, J. Bürck, J. Reichert, P. Wadhwani, O. Kukharenko, A.S. Ulrich. Homo- and heteromeric interaction strengths of the synergistic antimicrobial peptides PGLa and magainin 2 in membranes. Eur. Biophys. J. 46, 535–547 (2016)
J. Zerweck, E. Strandberg, J. Bürck, J. Reichert, P. Wadhwani, O. Kukharenko, A.S. Ulrich. Homo- and heteromeric interaction strengths of the synergistic antimicrobial peptides PGLa and magainin 2 in membranes. Eur. Biophys. J. 46, 535–547 (2016)
DOI: 10.1007/s00249-016-1120-7
 N. Zydziak, W. Konrad, F. Feist, S. Afonin, S. Weidner, C. Barner-Kowollik. Coding and decoding libraries of sequence-defined functional copolymers synthesized via photoligation. Nature Comm. 7, 13672 (2016)
N. Zydziak, W. Konrad, F. Feist, S. Afonin, S. Weidner, C. Barner-Kowollik. Coding and decoding libraries of sequence-defined functional copolymers synthesized via photoligation. Nature Comm. 7, 13672 (2016)
DOI: 10.1038/ncomms13672
Publications 2015
A. Grau-Campistany, E. Strandberg, P. Wadhwani, J. Reichert, J. Bürck, F. Rabanal and A. S. Ulrich. Hydrophobic mismatch demonstrated for membranolytic peptides, and their use as molecular rulers to measure bilayer thickness in native cells. Scientific Reports 5, 9388 (2015)
DOI:10.1038/srep09388
T. Asakura, T. Ohata, S. Kametani, K. Okushita, K. Yazawa, Y. Nishiyama, K. Nishimura, A. Aoki, F. Sizuki, H. Kaji, A. S. Ulrich, M. P. Williamson. Intermolecular packing in B. mori silk fibroin: Multinuclear NMR study of the model peptide (Ala-Gly)15 defines a heterogeneous antiparallel antipolar mode of assembly in the silk II form. Macromolecules 48, 28-36 (2015)
DOI: 10.1021/ma502191g
D. Bandak, O. Babii, R. Vasiuta, I. V. Komarov, P. K. Mykhailiuk. Design and synthesis of novel 19F-amino acid: A promising 19F NMR label for peptide studies. Org. Lett. 17, 226-229 (2015)
DOI:10.1021/ol503300m
M. Berditsch, S. Afonin, A. Steineker, N. Orel, I. Jakovkin, C. Weber and A. S. Ulrich. Fermentation and cost-effective 13C/15N labeling of the nonribosomal peptide gramicidin S for nuclear magnetic resonance structure analysis. Appl. Environ. Microbiol. 81, 3593-3603 (2015)
DOI:10.1128/AEM.00229-15
M. Berditsch, T. Jäger, N. Strempel, T. Schwartz, J. Overhage and A. S. Ulrich. Synergistic effect of membrane active peptides polymyxin B and gramicidin S on multidrug resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy 59, 5288-5296 (2015)
DOI:10.1128/AAC.00682-15
D. S. Blokhin, A. V. Filippov, O. N. Antzutkin, S. Afonin and V. V. Klochkov. Spatial structures of PAP(262 270) and PAP(274 284), two selected fragments of PAP(248 286), an enhancer of HIV infectivity. Appl. Magn. Reson. 46, 757-769 (2015)
DOI:10.1007/s00723-015-0669-0
J. Bürck, S. Roth, D. Windisch, P. Wadhwani, D. Moss and A. S. Ulrich. UV-CD12: synchrotron radiation circular dichroism beamline at ANKA. Journal of Synchrotron Radiation 22, 844-852 (2015)
DOI:10.1107/S1600577515004476
M. Cakici, Z.-G. Gu, M. Nieger, J. Bürck, L. Heinke and S. Bräse. Planar-chiral building blocks for metal-organic frameworks. Chem. Comm. 51, 4796-4798 (2015)
DOI: 10.1039/c5cc00694e
D. Helmer, I. Rink, J. A. R. Dalton, K. Brahm, M. Jöst, T. M. Nargang, W. Blum, P. Wadhwani, G. Brenner-Weiss, B. E. Rapp, J. Giraldo and K. Schmitz. Rational design of a peptide capture agent for CXCL8 based on a model of the CXCL8:CXCR1 complex. RSC Advances 5, 25657-25668 (2015)
DOI:10.1039/C4RA13749C
V. Kubyshkin, S. Afonin, S. Kara, N. Budisa, P. K. Mykhailiuk and A. S. Ulrich. gamma-(S)-Trifluoromethyl proline: Evaluation as a structural substitute of proline for solid state 19F-NMR peptide studies. Org. Biomol. Chem. 13, 3171-3181 (2015)
DOI:10.1039/C5OB00034C
G. Manzo, M. A. Scorciapino, P. Wadhwani, J. Bürck, N. P. Montaldo, M. Pintus, R. Sanna, M. Casu, A. Giuliani, G. Pirri, V. Luca, A. S. Ulrich and A. C. Rinaldi. Enhanced amphiphilic profile of a short ß-stranded peptide improves its antimicrobial activity. PLoS One 10, e0116379 (2015)
DOI:10.1371/journal.pone.0116379
J. Misiewicz, S. Afonin, S. L. Grage, J. van den Berg, E. Strandberg, P. Wadhwani and A. S. Ulrich. Action of the multifunctional peptide BP100 on native biomembranes examined by solid-state NMR. J. Biomol. NMR 61, 287-298 (2015)
DOI:10.1007/s10858-015-9897-8
J. Misiewicz, S. Afonin and A. S. Ulrich. Control and role of pH in peptide-lipid interactions in oriented membrane samples. Biochim. Biophys. Acta - Biomembranes 1848, 833-841 (2015)
DOI:10.1016/j.bbamem.2014.12.006
J.-D. Savoie, F. Otis, J. Bürck, A. S. Ulrich and N. Voyer. Crown ether helical peptides are preferentially inserted in lipid bilayers as a transmembrane ion channels. Biopolymers 104, 427-433 (2015)
DOI:10.1002/bip.22633
T. Serdiuk, I. Bakanovich, V. Lysenko, S. A. Alekseev, V. A. Skryshevsky, S. Afonin, E. Berger, A. Geloen and I. V. Komarov. Delivery of SiC-based nanoparticles into live cells driven by cell-penetrating peptides SAP and SAP-E. RSC Advances 5, 20498-20502 (2015)
DOI:10.1039/C4RA10688A
E. Strandberg, A. S. Ulrich. AMPs and OMPs: Is the folding and bilayer insertion of b-stranded outer membrane proteins governed by the same biophysical principles as for a-helical antimicrobial peptides? Biochim. Biophys. Acta - Biomembranes 1848, 1944-1954 (2015)
DOI:10.1016/j.bbamem.2015.02.019
E. Strandberg, J. Zerweck, D. Horn, G. Pritz, M. Berditsch, J. Bürck, P. Wadhwani and A. S. Ulrich. Influence of hydrophobic residues on the activity of the antimicrobial peptide magainin 2 and its synergy with PGLa. J. Pept. Sci. 21, 436-445 (2015)
DOI:10.1002/psc.2780
B. A. Wallace, J. Bürck. Third international synchrotron radiation circular dichroism spectroscopy meeting. Synchrotron Radiation News 28, 58-59 (2015)
DOI:10.1080/08940886.2015.1059247
D. Windisch, C. Ziegler, S. L. Grage, J. Bürck, M. Zeitler, P. L. Gor'kov, A. S. Ulrich. Hydrophobic mismatch drives the interaction of E5 with the transmembrane segment of PDGF receptor. Biophys. J. 109, 737-749 (2015)
DOI:10.1016/j.bpj.2015.07.022
H. Zamora-Carreras, B. Maestro, E. Strandberg, A. S. Ulrich, J. M. Sanz and M. Ángeles Jiménez. Micelle-triggered ß-hairpin to a-helix transition in a 14-residue peptide from a choline-binding repeat of the pneumococcal autolysin LytA. Chemistry - A European Journal 21, 8076-8089 (2015)
DOI:10.1002/chem.201500447
Publications 2014
 D. Aberle, C. Muhle-Goll, J. Bürck, M. Wolf, S. Reißer, B. Luy, W. Wenzel, A. S. Ulrich, G. Meyers. Structure of the membrane anchor of pestivirus glycoprotein Erns, a long tilted amphipathic helix. PLoS Pathogenes 10, e1003973 (2014)
D. Aberle, C. Muhle-Goll, J. Bürck, M. Wolf, S. Reißer, B. Luy, W. Wenzel, A. S. Ulrich, G. Meyers. Structure of the membrane anchor of pestivirus glycoprotein Erns, a long tilted amphipathic helix. PLoS Pathogenes 10, e1003973 (2014)
DOI: 10.1371/journal.ppat.1003973
 S. Afonin, R. W. Glaser, C. Sachse, J. Salgado, P. Wadhwani, A. S. Ulrich. 19F NMR screening of unrelated antimicrobial peptides shows that membrane interactions are largely governed by lipids. Biochim. Biophys. Acta - Biomembranes 1838, 2260-2268 (2014)
S. Afonin, R. W. Glaser, C. Sachse, J. Salgado, P. Wadhwani, A. S. Ulrich. 19F NMR screening of unrelated antimicrobial peptides shows that membrane interactions are largely governed by lipids. Biochim. Biophys. Acta - Biomembranes 1838, 2260-2268 (2014)
DOI: 10.1016/j.bbamem.2014.03.017
 O. Babii, S. Afonin, M. Berditsch, S. Reiβer S, P. K. Mykhailiuk, V. S. Kubyshkin, T. Steinbrecher, A. S. Ulrich, I. V. Komarov. Controlling biological activity with light: diarylethene-containing cyclic peptidomimetics. Angewandte Chem. Intl. Ed. 53, 3392-3395 (2014) and Angewandte Chemie 126, 3460-3463 (2014)
O. Babii, S. Afonin, M. Berditsch, S. Reiβer S, P. K. Mykhailiuk, V. S. Kubyshkin, T. Steinbrecher, A. S. Ulrich, I. V. Komarov. Controlling biological activity with light: diarylethene-containing cyclic peptidomimetics. Angewandte Chem. Intl. Ed. 53, 3392-3395 (2014) and Angewandte Chemie 126, 3460-3463 (2014)
DOI: 10.1002/anie.201310019 and 10.1002/ange.201310019
 C. G. Cranfield, B. A. Cornell, S. L. Grage, P. Duckworth, S. Carne, A. S. Ulrich, B. Martinac. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 106, 182-189 (2014)
C. G. Cranfield, B. A. Cornell, S. L. Grage, P. Duckworth, S. Carne, A. S. Ulrich, B. Martinac. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 106, 182-189 (2014)
DOI: 10.1016/j.bpj.2013.11.1121
 S. Fanghänel, P. Wadhwani, E. Strandberg, W. P. Verdurmen, J. Bürck, S. Ehni, P. K. Mykhailiuk, S. Afonin, D. Gerthsen, I. V. Komarov, R. Brock, A. S. Ulrich. Structure analysis and conformational transitions of the cell penetrating peptide transportan 10 in the membrane-bound state. PLoS ONE 9, e99653 (2014)
S. Fanghänel, P. Wadhwani, E. Strandberg, W. P. Verdurmen, J. Bürck, S. Ehni, P. K. Mykhailiuk, S. Afonin, D. Gerthsen, I. V. Komarov, R. Brock, A. S. Ulrich. Structure analysis and conformational transitions of the cell penetrating peptide transportan 10 in the membrane-bound state. PLoS ONE 9, e99653 (2014)
DOI: 10.1371/journal.pone.0099653
 U. I. M. Gerling, M. Salwiczek, C. D. Cadicamo, H. Erdbrink, C. Czekelius, S. L. Grage, P. Wadhwani, A. S. Ulrich, M. Behrends, G. Haufe, B. Koksch. Fluorinated amino acids in amyloid formation: A symphony of size, hydrophobicity and α-helix propensity. Chem. Sci. 5, 819-830 (2014)
U. I. M. Gerling, M. Salwiczek, C. D. Cadicamo, H. Erdbrink, C. Czekelius, S. L. Grage, P. Wadhwani, A. S. Ulrich, M. Behrends, G. Haufe, B. Koksch. Fluorinated amino acids in amyloid formation: A symphony of size, hydrophobicity and α-helix propensity. Chem. Sci. 5, 819-830 (2014)
DOI: 10.1039/C3SC52932K
 M. Girrbach, I. Meliciani, B. Waterkotte, S. Berthold, A. Oster, F. Brurein, T. Strunk, P. Wadhwani, S. Berensmeier, W. Wenzel, K. Schmitz. A fluorescence polarization assay for the experimental validation of an in silico model of the chemokine CXCL8 binding to receptor-derived peptides. Phys. Chem. Chem. Phys. 16, 8036-8043 (2014)
M. Girrbach, I. Meliciani, B. Waterkotte, S. Berthold, A. Oster, F. Brurein, T. Strunk, P. Wadhwani, S. Berensmeier, W. Wenzel, K. Schmitz. A fluorescence polarization assay for the experimental validation of an in silico model of the chemokine CXCL8 binding to receptor-derived peptides. Phys. Chem. Chem. Phys. 16, 8036-8043 (2014)
DOI: 10.1039/c3cp53850h
 S. L. Grage, X. Xu, M. Schmitt, P. Wadhwani, A. S. Ulrich. 19F-labeling of peptides revealing long-range NMR distances in fluid membranes. J. Phys. Chem. Lett. 5, 4256-4259 (2014)
S. L. Grage, X. Xu, M. Schmitt, P. Wadhwani, A. S. Ulrich. 19F-labeling of peptides revealing long-range NMR distances in fluid membranes. J. Phys. Chem. Lett. 5, 4256-4259 (2014)
DOI: 10.1021/jz502195t
 W. Grosse, G. Psakis, B. Mertins, P. Reiss, D. Windisch, F. Brademann, J. Bürck, A. S. Ulrich, U. Koert, L. O. Essen. Structure-based engineering of a minimal porin reveals loop-independent channel closure. Biochem. 53, 4826-4838 (2014)
W. Grosse, G. Psakis, B. Mertins, P. Reiss, D. Windisch, F. Brademann, J. Bürck, A. S. Ulrich, U. Koert, L. O. Essen. Structure-based engineering of a minimal porin reveals loop-independent channel closure. Biochem. 53, 4826-4838 (2014)
DOI: 10.1021/bi500660q
 Z. G. Gu, J. Bürck, A. Bihlmeier, J. Liu, O. Shekhah, P. G. Weidler, C. Azucena, Z. Wang, S. Heissler, H. Gliemann, W. Klopper, A. S. Ulrich, C. Wöll C. Oriented circular dichroism analysis of chiral surface-anchored metal-organic frameworks grown by liquid-phase epitaxy and upon loading with chiral guest compounds. Chemistry 20, 9879-9882 (2014)
Z. G. Gu, J. Bürck, A. Bihlmeier, J. Liu, O. Shekhah, P. G. Weidler, C. Azucena, Z. Wang, S. Heissler, H. Gliemann, W. Klopper, A. S. Ulrich, C. Wöll C. Oriented circular dichroism analysis of chiral surface-anchored metal-organic frameworks grown by liquid-phase epitaxy and upon loading with chiral guest compounds. Chemistry 20, 9879-9882 (2014)
DOI: 10.1002/chem.201402567
 J. Podlech, S. C. Fleck, M. Metzler, J. Bürck, A. S. Ulrich. Determination of the absolute configuration of perylene quinone-derived mycotoxins by measurement and calculation of electronic circular dichroism spectra and specific rotations. Chemistry 20, 11463-11470 (2014)
J. Podlech, S. C. Fleck, M. Metzler, J. Bürck, A. S. Ulrich. Determination of the absolute configuration of perylene quinone-derived mycotoxins by measurement and calculation of electronic circular dichroism spectra and specific rotations. Chemistry 20, 11463-11470 (2014)
DOI: 10.1002/chem.201402567
 S. Reißer, E. Strandberg, T. Steinbrecher, A. S. Ulrich. 3D hydrophobic moment vectors as a tool to characterize the surface polarity of amphiphilic peptides. Biophys. J. 106, 2385-2394 (2014)
S. Reißer, E. Strandberg, T. Steinbrecher, A. S. Ulrich. 3D hydrophobic moment vectors as a tool to characterize the surface polarity of amphiphilic peptides. Biophys. J. 106, 2385-2394 (2014)
DOI: 10.1016/j.bpj.2014.04.020
 S. Ruskamo, R. P. Yadav, S. Sharma, M. Lehtimäki, S. Laulumaa, S. Aggarwal, M. Simons, J. Bürck, A. S. Ulrich, A. H. Juffer, I. Kursula, P. Kursula. Atomic resolution view into the structure-function relationships of the human myelin peripheral membrane protein P2. Acta Cryst. D 70, 165-176 (2014)
S. Ruskamo, R. P. Yadav, S. Sharma, M. Lehtimäki, S. Laulumaa, S. Aggarwal, M. Simons, J. Bürck, A. S. Ulrich, A. H. Juffer, I. Kursula, P. Kursula. Atomic resolution view into the structure-function relationships of the human myelin peripheral membrane protein P2. Acta Cryst. D 70, 165-176 (2014)
DOI: 10.1107/S1399004713027910
 L. A. Sommer, J. J. Janke, W. F. Bennett, J. Bürck, A. S. Ulrich, D. P. Tieleman, S. A. Dames. Characterization of the immersion properties of the peripheral membrane anchor of the FATC domain of the kinase "target of rapamycin" by NMR, oriented CD spectroscopy, and MD simulations. J. Phys. Chem. B 118, 4817-4831 (2014)
L. A. Sommer, J. J. Janke, W. F. Bennett, J. Bürck, A. S. Ulrich, D. P. Tieleman, S. A. Dames. Characterization of the immersion properties of the peripheral membrane anchor of the FATC domain of the kinase "target of rapamycin" by NMR, oriented CD spectroscopy, and MD simulations. J. Phys. Chem. B 118, 4817-4831 (2014)
DOI: 10.1021/jp501533d
 E. Strandberg, A. S. Ulrich. Dynamic structure analysis of peptides in membranes by solid-state NMR. In "Advances in Biological Solid-State NMR: Proteins and Membrane-Active Peptides”, Topics in Current Chemistry, Book 306, pages 304-319, Editors: F. Separovic, A. Naito, Royal Society of Chemistry (2014)
E. Strandberg, A. S. Ulrich. Dynamic structure analysis of peptides in membranes by solid-state NMR. In "Advances in Biological Solid-State NMR: Proteins and Membrane-Active Peptides”, Topics in Current Chemistry, Book 306, pages 304-319, Editors: F. Separovic, A. Naito, Royal Society of Chemistry (2014)
ISBN: 9781849739108
http://pubs.rsc.org/en/content/ebook/978-1-84973-910-8#!divbookcontent
 A. N. Tkachenko, P. K. Mykhailiuk, D. S. Radchenko, O. Babii, S. Afonin, A. S. Ulrich, I. V. Komarov. Design and synthesis of a monofluoro-substituted aromatic amino acid as a conformationally restricted 19F NMR label for membrane-bound peptides. Eur. J. Org. Chem. 2014, 3584-3591 (2014)
A. N. Tkachenko, P. K. Mykhailiuk, D. S. Radchenko, O. Babii, S. Afonin, A. S. Ulrich, I. V. Komarov. Design and synthesis of a monofluoro-substituted aromatic amino acid as a conformationally restricted 19F NMR label for membrane-bound peptides. Eur. J. Org. Chem. 2014, 3584-3591 (2014)
DOI: 10.1002/ejoc.201301737
 D. Volz, M. Wallesch, S. L. Grage, J. Göttlicher, R. Steininger, D. Batchelor, T. Vitova, A. S. Ulrich, C. Heske, L. Weinhardt, T. Baumann, S. Bräse. Labile or stable: Can homoleptic and heteroleptic pyrphos-copper complexes be processed from solution? Inorg. Chem. 53, 7837-7847 (2014)
D. Volz, M. Wallesch, S. L. Grage, J. Göttlicher, R. Steininger, D. Batchelor, T. Vitova, A. S. Ulrich, C. Heske, L. Weinhardt, T. Baumann, S. Bräse. Labile or stable: Can homoleptic and heteroleptic pyrphos-copper complexes be processed from solution? Inorg. Chem. 53, 7837-7847 (2014)
DOI: 10.1021/ic500135m
 P. Wadhwani, E. Strandberg, J. van den Berg, C. Mink, J. Bürck, R. A. Ciriello, A. S. Ulrich. Dynamical structure of the short multifunctional peptide BP100 in membranes. Biochim. Biophys. Acta - Biomembranes 1838, 940-949 (2014)
P. Wadhwani, E. Strandberg, J. van den Berg, C. Mink, J. Bürck, R. A. Ciriello, A. S. Ulrich. Dynamical structure of the short multifunctional peptide BP100 in membranes. Biochim. Biophys. Acta - Biomembranes 1838, 940-949 (2014)
DOI: 10.1016/j.bbamem.2013.11.001
 T. H. Walther, A. S. Ulrich. Transmembrane helix assembly and the role of salt bridges. Curr. Op.Struct. Biol. 27, 63-68 (2014)
T. H. Walther, A. S. Ulrich. Transmembrane helix assembly and the role of salt bridges. Curr. Op.Struct. Biol. 27, 63-68 (2014)
DOI: 10.1016/j.sbi.2014.05.003
 Y. Wang, T. Zhao, D. Wei, E. Strandberg, A. S. Ulrich, J. P. Ulmschneider. How reliable are molecular dynamics simulations of membrane active antimicrobial peptides? Biochim. Biophys. Acta - Biomembranes 1838, 2280-2288
Y. Wang, T. Zhao, D. Wei, E. Strandberg, A. S. Ulrich, J. P. Ulmschneider. How reliable are molecular dynamics simulations of membrane active antimicrobial peptides? Biochim. Biophys. Acta - Biomembranes 1838, 2280-2288
DOI: 10.1016/j.bbamem.2014.04.009
 D. Windisch, C. Ziegler, J. Bürck, A. S. Ulrich. Structural characterization of a C-terminally truncated E5 oncoprotein from papillomavirus in lipid bilayers. Biol. Chem. 395, 1443-1452 (2014)
D. Windisch, C. Ziegler, J. Bürck, A. S. Ulrich. Structural characterization of a C-terminally truncated E5 oncoprotein from papillomavirus in lipid bilayers. Biol. Chem. 395, 1443-1452 (2014)
DOI: 10.1515/hsz-2014-0222
| Titel | Autor | Quelle |
|---|---|---|
| Interaction of prostatic acid phosphatase fragments with a lipid bilayer as studied by NMR spectroscopy | Filippov, A.; Khakimov, A.; Afonin, S.; Antzutkin, O.N. | Mendeleev Communications, 23, 313-315 |
| Lipid membrane association of myelin proteins and peptide segments studied by oriented and synchrotron radiation circular dichroism spectroscopy | Muruganandam, G., J. Bürck, A.S. Ulrich, I. Kursula, P. Kursula | J. Phys. Chem. B, 117, 14983-14993 |
| Incorporation of labile trans-4,5-difluoromethanoproline into a peptide as a stable label for 19F-NMR structure analysis | Kubyshkin, V.S., P.K. Mykhailiuk, S. Afonin, S.L. Grage, I.V. Komarov, A.S. Ulrich | J. Fluorine Chem., 152, 136-143 |
| Transformation of the matrix structure of shrimp shells during bacterial deproteination and demineralization | Xu Y, Bajaj M, Schneider R, Grage SL, Ulrich AS, Winter J, Gallert C | Microb Cell Fact., 12(1):90 |
| Curvature engineering: positive membrane curvature induced by epsin N-terminal peptide boosts internalization of octaarginine | Pujals S, Miyamae H, Afonin S, Murayama T, Hirose H, Nakase I, Taniuchi K, Umeda M, Sakamoto K, Ulrich AS, Futaki S | ACS Chem Biol., 8(9):1894-9 |
| Cell surface clustering of heparan sulfate proteoglycans by amphipathic cell-penetrating peptides does not contribute to uptake | Verdurmen WP, Wallbrecher R, Schmidt S, Eilander J, Bovee-Geurts P, Fanghänel S, Bürck J, Wadhwani P, Ulrich AS, Brock R | J Control Reease., 170(1):83-91 |
| Stereochemical effects on the aggregation and biological properties of the fibril-forming peptide [KIGAKI]3 in membranes | Wadhwani P, Reichert J, Strandberg E, Bürck J, Misiewicz J, Afonin S, Heidenreich N, Fanghänel S, Mykhailiuk PK, Komarov IV, Ulrich AS | Phys Chem Chem Phys., 15(23):8962-71 |
| Design, synthesis, and application of a trifluoromethylated phenylalanine analogue as a label to study peptides by solid-state 19F NMR spectroscopy | Tkachenko AN, Radchenko DS, Mykhailiuk PK, Afonin S, Ulrich AS, Komarov IV |
Angew Chem Int Ed Engl., 52(25):6504-7. Angew Chem., 125(25):6632-5. |
| Canonical azimuthal rotations and flanking residues constrain the orientation of transmembrane helices | Sánchez-Muñoz OL, Strandberg E, Esteban-Martín E, Grage SL, Ulrich AS, Salgado J | Biophys J., 104(7):1508-16 |
| Synergistic insertion of antimicrobial magainin-family peptides in membranes depends on the lipid spontaneous curvature | Strandberg E, Zerweck J, Wadhwani P, Ulrich AS | Biophys J., 104(6):L9-11 |
| Resemblance of electrospun collagen nanofibers to their native structure | Bürck J, Heissler S, Geckle U, Ardakani MF, Schneider R, Ulrich AS, Kazanci M. | Langmuir, 29(5):1562-72 |
| Nanocrystalline solid solutions AlySn1-yO2-y/2 (y=0.57, 0.4) as electrode materials for lithium-ion batteries * | Becker SM, Issac I, Heinzmann R, Scheuermann M, Eichhofer A, Wang D, Chakravadhanula VSK, Kubel C, Ulrich AS, Hahn H, Indris S J | Power Sources, 229: 149-158 |
| Folding and self-assembly of the TatA translocation pore based on a charge zipper mechanism |
Walther, T.H., C. Gottselig, S.L. Grage, M. Wolf, A.V. Vargiu, M.J. Klein, S. Vollmer, S. Prock, M. Hartmann, S. Afonin, E. Stockwald, H. Heinzmann, O.V. Nolandt, W. Wenzel, P. Ruggerone, A.S. Ulrich |
Cell, 152; 316-326 |
| A 19F NMR label to substitute polar amino acids in peptides: a CF3-substituted analogue of serine and threonine |
Tkachenko, A.N., P.K. Mikhailiuk S. Afonin, D.S. Radchenko, V.S. Kubyshkin, A.S. Ulrich, I.V. Komarov |
Angew. Chem. Int. Ed., 52; 1486-1489 Angew.Chem., 125; 1526-1529 |
| Multilayered core-shell structure of polyol-stabilized CaF2 nanoparticles characterized by NMR |
Witter, R., M. Roming, C. Feldmann, A.S. Ulrich |
J. Coll. Interface Sci., 390, 259-257 |
| Spatial structure of heptapeptide Glu-Ile-Leu-Asn-His-Met-Lys, a fragment of the HIV enhancer prostatic acid phosphatase, in aqueous and SDS micelle solutions |
Blochin, D.S., O.V. Aganova, A.R. Yulmetov, A.V. Filippov, B.I. Gizatullin, S. Afonin, O.N. Antzutkin, V.V. Klochkov |
J. Mol. Struct., 1033, 59-66 |
| Titel | Autor | Quelle |
|---|---|---|
| Synthesis of nanocrystalline solid solutions AlySn1-yO2-y/2 (y = 0.57, 0.4) investigated by XRD, 27Al/119Sn MAS NMR and Mössbauer spectroscopy |
Issac, I., R. Heinzmann, S.M. Becker, Th. Bräuninger, Zh. Zhao-Karger, C. Adelhelm, V.S. Kiran Chakravadhanula, Ch. Kübel, A.S. Ulrich, S. Indris |
RSC Adv., 2:10700-10707 |
| Incorporation of cis- and trans-4,5-difluoromethanoprolines into polypeptides |
Kubyshkin, V.S., P.K. Mykhailiuk, S. Afonin, A.S. Ulrich, I.V. Komarov |
Org. Lett., 14, 5811 |
| Spatial structure of heptapeptide Aβ16-22 (beta-amyloid Aβ1-40 active fragment) in solution and in complex with a biological membrane model |
Usachev, K.S., S.V. Efimov, A.R. Yulmetov, A.V. Filippov, O.N. Antzutkin, O.N., S. Afonin, V.V. Klochkov |
Magn. Res. Chem., 50, 784-792 |
| Rapid calculation of protein chemical shifts using bond polarization theory and its application to protein structure refinement |
Jakovkin, I., M. Klipfel, C. Muhle-Goll, A.S. Ulrich, B. Luy, U. Sternberg |
Phys. Chem. Chem. Phys., 14, 12263-12276 |
| Self-assembly of flexible β-strands into immobile amyloid-like β-sheets in membranes as revealed by solid-state 19F NMR |
Wadhwani, P., E. Strandberg, N. Heidenreich, J. Bürck, S. Fanghänel, A.S. Ulrich |
J. Am. Chem. Soc., 134, 6512-6515 |
| Antimicrobial peptides can enhance the risk of persistent infections |
Berditsch, M., S. Afonin, T. Vladimirova, P. Wadhwani, A.S. Ulrich |
Front. Immunol., 3, 222:1-4 |
| Hydrophobic matching controls the tilt and stability of the dimeric platelet-derived growth factor receptor (PDGFR) β transmembrane segment |
Muhle-Goll, C., S. Hoffmann, S. Afonin, S.L. Grage, A.A. Polyanski, D. Windisch, M. Zeitler, J. Bürck, A.S. Ulrich |
J. Biol. Chem., 287, 26178-26186 |
| Alignment of druglike compounds in lipid bilayers analyzed by solid-state 19F-NMR and molecular dynamics, based on dipolar couplings of adjacent CF3 groups | Dürr, U.H.N., S. Afonin, B. Hoff, G. de Luca, J.W. Emsley, A.S. Ulrich |
J. Phys. Chem. B, 116, 4769-4782 |
| Membrane-active peptides and the clustering of anionic lipids |
Wadhwani, P., R.F. Epand, N. Heidenreich, J. Bürck, A.S. Ulrich, R.M. Epand |
Biophys. J., 103, 265-274 |
| A peptidic unconjugated GRP78/BiP ligand inhibits unfolded protein response and induces prostate cancer cell death |
Maddalo, D., A. Neeb, K. Jehle, K. Schmitz, L. Shatkina, C. Muhle-Goll, L. Shatkina, T. Walther, A. Bruchmann, S.M. Gopal, W. Wenzel, A.S. Ulrich, A.C.B. Cato |
PloS ONE 7, e45690 |
| A new type of intracellular retention signal identified in a pestivirus structural glycoprotein |
Burrack, S., D. Aberle, J. Bürck, A.S. Ulrich, G. Meyers |
FASEB J., 26, 3292-3305 |
| Reorientation and dimerization of the membrane-bound antimicrobial peptide PGLa from microsecond all-atom MD simulations |
Ulmschneider, J.P., J.C. Smith, M.B. Ulmschneider, A.S. Ulrich, E. Strandberg |
Biophys. J., 103, 472-482 |
| Peptide-lipid interactions of the stress-response peptide TisB that induces bacterial persistence | Steinbrecher, Th., S. Prock, J. Reichert, P. Wadhwani, B. Zimpfer, J. Bürck, M. Berditsch, M. Elstner, A.S. Ulrich |
Biophys. J., 103, 1460-1469 |
| Structure analysis of the membrane-bound PhoD signal peptide of the Tat translocase shows an N-terminal amphiphilic helix |
Klein, M.J., S.L. Grage, C. Muhle-Goll, J. Bürck, S. Afonin, A.S. Ulrich |
Biochim. Biophys. Acta, 1818, 3025-3031 |
| Comparative analysis of transmembrane peptides using solid-state 2H- and 15N-NMR: mobility matters |
Grage, S.L., E. Strandberg, P. Wadhwani, S. Esteban-Martín, J. Salgado, A.S. Ulrich |
Eur. Biophys. J., 41, 475-482 |
| Lipid shape is a key factor for membrane interactions of amphipathic helical peptides |
Strandberg E., D. Tiltak, S. Ehni, P. Wadhwani, A.S. Ulrich |
Biochim. Biophys. Acta, 1818: 1764-1776 |
| Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat |
Paulmann, M., Th. Arnold, D. Linke, S. Özdirekcan, A. Kopp, Th. Gutsmann, H. Kalbacher, I. Wanke, V.J. Schuenemann, M. Habeck, J. Bürck, A.S. Ulrich, B. Schittek |
J. Biol. Chem., 287, 8434-8443 |
| A novel dendrimeric peptide with antimicrobial properties: structure-function analysis of SB056 |
Scorciapino, M.A., G. Pirri, A.V. Vargiu, P. Ruggerone, A. Giuliani, M. Casu, J. Buerck, P. Wadhwani, A.S. Ulrich, A.C. Rinaldi |
Biophys. J, 102, 1039-1048 |
| Hydrophobic mismatch of mobile transmembrane helices: Merging theory and experiments |
Strandberg, E., S. Esteban-Martín, A.S. Ulrich, J. Salgado |
Biochim. Biophys. Acta, 1818, 1242-1249 |
| Non-equilibrium structure of Zn2SnO4 spinel nanoparticles |
Šepelák, S., S.M. Becker, I. Bergmann, S. Indris, M. Scheuermann, A. Feldhoff, C. Kübel, M. Bruns, N. Stürzl, A.S. Ulrich, M. Ghafari, C.P. Grey, K.D. Becker, P. Heitjans |
J. Mater. Chem., 22, 3117-3126 |
| Magnetically oriented dodecylphosphocholine bicelles for solid-state NMR structure analysis |
Nolandt, O.V., T.H. Walther, S.L. Grage, A.S. Ulrich |
Biochim. Biophys. Acta, 1818, 1142-1147 |
| Electrochemical insertion of lithium in mechanochemically synthesized Zn2SnO4 |
Becker, S.M., M. Scheuermann, V. Šepelák, A. Eichhöfer, Di Chen, R. Mönig, A.S. Ulrich, H. Hahn, S. Indris |
Phys. Chem. Chem. Phys., 13, 19624-19631 |
| Antimicrobial and cell-penetrating peptides induce lipid vesicle fusion by folding and aggregation |
Wadhwani, P., J. Reichert, J. Bürck, A.S. Ulrich |
Eur. Biophys. J., 41, 177-187 |
| Anisotropic organization and microscopic manipulation of self-assembling synthetic porphyrin micro-rods that mimic chlorosomes: bacterial light-harvesting systems |
Chappaz-Gillot, C., P.L. Marek, B.J. Blaive, G. Canard, J. Bürck, G. Garab, H. Hahn, T. Jávorfi, L. Kelemen, R. Krupke, D. Mössinger, P. Ormos, C.M. Reddy, C. Roussel, G. Steinbach, M. Szabó, A.S. Ulrich, N. Vanthuyne, A. Vijayaraghavan, A. Zupcanova, T.S. Balaban |
J. Am. Chem. Soc. 134, 944-954 |
| Trifluoromethyl-substituted α-amino acids as solid state 19F-NMR labels for structural studies of membrane-bound peptides | Kubyshkin VS, Komarov IV, Afonin S, Mykhailiuk PK, Grage SL, Ulrich AS | “Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications” ed.: Gouverneur V, Müller K; Imperial Collage Press, 91-138 |
| Solid-state 19F-NMR of peptides in native membranes | Koch K, Afonin S, Ieronimo M, Berditsch M, Ulrich AS | Topics in Current Chemistry, 306, 89-119 |
| Titel | Autor | Quelle |
|---|---|---|
| Click chemistry produces hyper-cross-linked polymers with tetrahedral cores | Plietzsch O, Schilling CI, Grab T, Grage SL, Ulrich AS, Comotti A, Sozzani P, Muller T, Bräse S | New Journal of Chemistry, 15, 1577-1581 |
| Preferential uptake of arginine-rich L- versus D-amino acid cell-penetrating peptides in a cell type-dependent manner | Verdurmen WPR, Bovee-Geurts P, Wadwhani P, Ulrich AS, Hällbrink M, van Kuppevelt T, Brock R | Chemistry and Biology, 18, 1000-1010 |
| Charge isomers of myelin basic protein: structure and interactions with membranes, nucleotide analogues, and calmodulin | Wang C, Neugebauer U, Bürck J, Myllykoski M, Baumgärtel P, Popp J, Kursula P. | PLoS One 6, e199156433-6437 |
| Local structural disorder and relaxation in SnO2 nanostructures studied by 119Sn MAS NMR and 119Sn mössbauer spectroscopy | Indris S, Scheuermann M, Becker SM, Šepelák V, Kruk R, Suffner J, Gyger F, Feldmann C, Ulrich AS, Hahn H | Journal of Physical Chemistry C (2011) 115(14):6433-6437 |
| Special issue on membrane-active peptides | Afonin S, Juretić D, Separovic F, Ulrich AS. | Eur. Biophys. J., 40 (4): 347-348 |
| Bilayer-mediated clustering and functional interaction of MscL channels | Grage SL, Keleshian AM, Turdzeladze T, Battle AR, Tay WC, May RP, Holt SA, Contera SA, Haertlein M, Moulin M, Pal P, Rohde PR, Forsyth VT, Watts A, Huang KC, Ulrich AS, Martinac B | Biophys. J., 100(5):1252-1260 |
| Variable angle NMR spectroscopy and its application to the measurement of residual chemical shift anisotropy | Kummerlöwe G, Grage SL, Thiele CM, Kuprov I, Ulrich AS, Luy B | Journal of Magnetic Resonance (2011); 209(1):19-30 |
| Using the peptide Bp100 as a cell-penetrating tool for the chemical engineering of actin filaments within living plant cells | Eggenberger K, Mink C, Wadhwani P, Ulrich AS, Nick P | ChemBioChem (2011); 12(1):132-137 |
| Fluorescence of phytochrome adducts with synthetic locked chromophores | Zienicke B, Chen LY, Khawn H, Hammam MA, Kinoshita H, Reichert J, Ulrich AS, Inomata K, Lamparter T | Journal of Biological Chemistry (2011); 286(2): 1103-1113 |
| A kinked antimicrobial peptide from Bombina maxima. I. Three-dimensional structure determined by NMR in membrane-mimicking environments | Toke O, Bánóczi Z, Király P, Heinzmann R, Bürck J, Ulrich AS, Hudecz F | Eur. Biophys. J., 40 (4): 447-462 |
| A kinked antimicrobial peptide from Bombina maxima. II. Behavior in phospholipid membrane | Heinzmann R, Grage SL, Schalck C, Bürck J, Bánóczi Z, Toke O, Ulrich AS | Eur. Biophys. J., 40 (4): 463-470 |
| Irregular structure of the HIV fusion peptide in membranes demonstrated by solid state NMR and MD simulations | Grasnick, D.; Sternberg, U.; Strandberg, E.; Wadhwani, P.; Ulrich, A.S. | Eur. Biophys. J., 40 (4): 529-543 |
| Titel | Autor | Quelle |
|---|---|---|
| Membrane alignment of the pore-forming component TatA(d) of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy | Walther TH, Grage SL, Roth N, Ulrich AS | J. Am. Chem. Soc., 132(45):15945-159563 |
| Compatibility of the conformationally rigid CF3 Bpg side chain with the hydrophobic coiled coil interface | Salwiczek M, Mikhailiuk, PK, Afonin S, Komarov IV, Ulrich AS, Koksch B | Amino Acids (2010); 39(5):1589-1593 |
| Ridge-tile-like chiral topology: synthesis, resolution, and complete chiroptical characterization of enantiomers of edge-sharing binuclear square planar complexes of Ni(II) bearing achiral ligands | Soloshonok VA, Ono T, Ueki H, Vanthuyne N, Balaban TS, Bürck J, Fliegl H, Klopper W, Naubron JV, Bui TT, Drake AF, Roussel C | J. Am. Chem. Soc., 132(30):10477-10483 |
| Short cationic antimicrobial peptides interact with ATP | Hilpert K, McLeod B, Yu J, Elliott MR, Rautenbach M, Ruden S, Bürck J, Muhle-Goll C, Ulrich AS, Keller S, Hancock REW | Antimicrobial Agents and Chemotherapy (2010); 54(10):4480-4483 |
| Structural role of the conserved cysteines in the dimerization of the viral transmembrane oncoprotein E5 | Windisch D, Hoffmann S, Afonin S, Vollmer S, Benamira S, Langer B, Bürck J, Muhle-Goll C, Ulrich AS | Biophys. J., 99(6):1764-1772 |
| Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa revealed by transmission and scanning electron microscopy | Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS | Antimicrobial Agents and Chemotherapy (2010); 54(8):3132-3142 |
| 19F-NMR analysis of the antimicrobial peptide PGLa bound to native cell membranes from bacterial protoplasts and human erythrocytes | Ieronimo M, Afonin S, Koch K, Berditsch M, Wadhwani P, Ulrich AS | J. Am. Chem. Soc., 132(26):8822-8824 |
| The conserved Cys76 plays a crucial role for the conformation of reduced glutathione peroxidase-type tryparedoxin peroxidase | Muhle-Goll C, Füller F, Ulrich AS, Krauth-Siegel RL |
FEBS Letters (2010); 584(5):1027-1032 |
| Solid state NMR analysis of peptides in membranes: influence of dynamics and labeling scheme | Esteban-Martín S, Strandberg E, Salgado J, Ulrich AS |
Biochim. Biophys. Acta - Biomembranes, 1798(2), 252-257
|
| An optimized protocol for the multigram synthesis of 3-(trifluoromethyl)bicyclopent-[1.1.1]-1-ylglycine (CF3-Bpg)” | Mykhailiuk PK, Voievoda NM, Afonin S, Ulrich AS, Komarov IV | Journal of Fluorine Chemistry (2010); 131(2):217-220 |
| Dynamic transitions of membrane active peptides | Grage SL, Afonin S, Ulrich AS | “Antimicrobial Peptides. Methods and Protocols” |
| Titel | Autor | Quelle |
|---|---|---|
| A cell-penetrating peptide derived from human lactoferrin with conformation-dependent uptake efficiency | Duchardt F, Ruttekolk IR, Verdurmen WPR, Lortat-Jacob H, Bürck J, Hufnagel H, Fischer R, van den Heuvel M, Lowik DWPM, Vuister GW, Ulrich A, de Waard M, Brock R | Journal of Biological Chemistry (2009); 284 (52), 36099-36108 |
| Chemical labeling strategy with (R)- and (S)-trifluoromethylalanine for solid state 19F-NMR analysis of peptaibols in membranes | Maisch D, Wadhwani P, Afonin S, Böttcher C, Koksch B, Ulrich AS |
J. Am. Chem. Soc., 131(43):15596-15597
|
| Synthesis of a conformationally rigid analogue of 2-aminoadipic acid ¬containing an 8-azabicyclo[3.2.1]octane skeleton | Kubyshkin VS, Mykhailiuk PK, Ulrich AS, Komarov IV | Synthesis-Stuttgart (2009); 19:3327-3331 |
| Calculation of fluorine chemical shift tensors for the interpretation of oriented 19F-NMR spectra of gramicidin A in membranes | Sternberg U, Klipfel M, Grage SL, Witter R, Ulrich AS |
Phys. Chem. Chem. Phys., 11(32):7048-7060
|
| Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides | Ruden S, Hilpert K, Berditsch M, Wadhwani P, Ulrich AS | Antimicrobial Agents and Chemotherapy (2009); 53(8):3538-3540 |
| Orientation and dynamics of peptides in membranes calculated from 2H-NMR data | Strandberg E, Esteban-Martin S, Salgado J, Ulrich AS |
Biophys. J., 96(8): 3223-3232
|
| Structure and dynamics of the human muscle LIM protein | Schallus T, Fehér K, Ulrich AS, Stier G, Muhle-Goll C |
FEBS Letters; 583(6):1017-1022 |
| Synergistic transmembrane insertion of the heterodimeric PGLa/magainin 2 complex studied by solid-state NMR | Strandberg E, Tremouilhac P, Wadhwani P, Ulrich AS |
Biochim. Biophys. Acta - Biomembranes; 1788(8): 1667-1679 |
| Influence of whole-body dynamics on 15N PISEMA NMR spectra of membrane proteins: A theoretical analysis | Esteban-Martin S, Strandberg E, Fuertes G, Ulrich AS, Salgado J |
Biophys. J., 96(8): 3233-3241 |
| Screening and characterization of surface-tethered cationic peptides for antimicrobial activity | Hilpert K, Elliott M, Jenssen H, Kindrachuk J, Fjell CD, Körner J, Winkler DFH, Weaver LL, Henklein P, Ulrich AS, Chiang SHY, Farmer SW, Pante N, Volkmer R,Hancock REW |
Chemistry and Biology; 16(1): 58-69 |
| Structure analysis of membrane-active peptides using 19F-labeled amino acids and solid state NMR | Wadhwani P.; Strandberg E. |
Fluorine in Medicinal Chemistry and Chemical Biology, (ed.: Ojima I.), Wiley-Vch; pp: 463-493 |
| Mimics of the Self-Assembling Chlorosomal Bacteriochlorophylls: Regio- and Stereoselective Synthesis and Stereoanalysis of Acyl(1-hydroxyalkyl)porphyrins | Balaban T.S.; Bhise A.D.; Bringmann G.; Buerck J. ; Chappaz-Gillot C.; Eichhoefer A.; Fenske D.; Goetz D.C.G.; Knauer M.; Mizoguchi T.; Moessinger D.; Roesner H.; Roussel C.; Schraut M.; Tamiaki H.; Vanthuyne N. |
J. Am. Chem. Soc., 131, 14480-14492 |
| Structure analysis of the membrane protein TatCd from the Tat system of B. subtilis by circular dichroism | Nolandt O.V.; Walther T.H.; Roth S.; Bürck J.;Ulrich A.S. |
Biochim. Biophys. Acta, 1788, 2238-2244
|
| Constrained Synthesis and Organization of Catalytically Active Metal Nanoparticles by Self-Assembled Protein Templates | Behrens S.; Heyman A.; Maul R.; Essig S.; Steigerwald S.; Quintilla A.; Wenzel W.; Bürck J.; Dgany O.; Shoseyov O. |
Adv. Mater., 21, 3515-3519 |
| Titel | Autor | Quelle |
|---|---|---|
| Solid state 19F-NMR analysis of oriented biomembranes | A.S. Ulrich | Modern Magnetic Resonance (ed. G.A.Webb), Springer; 257-263 |
| Crystal structure refinement using chemical shifts | U. Sternberg, R. Witter, A.S. Ulrich | Modern Magnetic Resonance (ed. G.A.Webb), Springer; 67-74 |
| Application of REDOR for distance measurements in biological solids | S.L. Grage, A. Watts |
Ann. Rep. NMR Spec., 60, 191-228 |
| Structure analysis of the protein translocating channel TatA in membranes using a multi-construct approach | C. Lange, S.D. Müller, T.H. Walther, J. Bürck, A.S. Ulrich |
Biochim. Biophys. Acta; 1768; 2627-2634
|
| A critical evaluation of the conformational requirements of fusogenic peptides in membranes | J. Reichert, D. Grasnick, S. Afonin, J. Bürck, P. Wadhwani, A.S. Ulrich | Eur.J.Biophys. 2007; 36; 405-413 |
| Using fluorinated amino acids for structure analysis of membrane-active peptides by solid-state 19F-NMR | P. Wadhwani, P. Tremouilhac, E. Strandberg, S. Afonin, S.L. Grage, M. Ieronimo, M. Berditsch, A.S. Ulrich | "Current Fluoroorganic Chemistry" (eds. V.Soloshonok, K.Mikami, T.Yamazaki, J.T.Welch & J.Honek); American Chemical Society, Washington,DC. 2007, Chapter 27, 431-446 |
| Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides | E. Strandberg, D. Tiltak, M. Ieronimo, N. Kanithasen, P. Wadhwani, A.S. Ulrich | Pure and Appl. Chem. 2007; 79, 717-728 |
| Evaluating the amino acid CF3-bicyclopentylglycine as a new label for solid-state F19-NMR structure analysis of membrane-bound peptides | S. Afonin, P.K. Mikhailiuk, I.V. Komarov & A.S. Ulrich | J. Pept. Sci. 13: 614-623 |
| Circular dichroism analysis of penicillin G acylase covalently immobilized on silica nanoparticles | B. Kranz, J. Bürck, M. Franzreb, R. Köster & A.S. Ulrich | J. Coll. Interface Sci. 316: 413–419 |
| All-atom Molecular Dynamics simulations of membrane-bound molecules with NMR orientational constraints | U. Sternberg, R. Witter & A.S. Ulrich | J. Biomol. NMR 38: 23-39 |
| The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation | M. Berditsch, S. Afonin & A.S. Ulrich | Appl. Environm. Microbiol., 73, 6620-6628 |
| Low-E probe for 19F-1H NMR of dilute biological samples | P.L. Gork’ov, R. Witter, E.Y. Chekmenev, F. Nozirov, R. Fu & W.W. Brey | J. Magn. Res., 190, 17-24 |
| Structural characterization of the pore forming protein TatAd of the twin-arginine translocase in membranes by solid-state 15N-NMR, | S.D. Müller , A.A. De Angelis, T.H. Walther, S.L. Grage, C. Lange, S.J. Opella & A.S. Ulrich |
Biochim. Biophys. Acta, 1768, 3071-3079 |
| Titel | Autor | Quelle |
|---|---|---|
| Solid state 19F-nuclear magnetic resonance analysis of membrane-active peptides | A.S.Ulrich, P.Wadhwani, U.H.N.Dürr, S.Afonin, R.W.Glaser, E.Strandberg, P.Tremouilhac, C.Sachse, M.Berditchevskaia, S.Grage | NMR Spectroscopy of Biological Solids (Ed. A. Ramamoorthy), Taylor & Francis; 215-236 |
| Solid state 19F-NMR methods for studying biomembranes | A.S.Ulrich | Prog. NMR Spectr. 2005; 46: 1-21 |
| Concentration-dependent re-alignment of the peptide PGLa in lipid membranes observed by solid state 19F-NMR | R.W.Glaser, C.Sachse, U.H.N.Dürr,P.Wadhwani, S.Afonin, E.Strandberg, A.S.Ulrich | Biophys. J. 2005; 88: 3392-3397 |
| 2H-NMR study and molecular dynamics simulation of the location, alignment and mobility of pyrene in POPC bilayers | B.Hoff, E.Strandberg, A.S.Ulrich, P.Tielemann, C.Posten | Biophys. J. 2005; 88: 1818-1827 |
Earlier publications
2004:
- U.Lauf, A.Fahr, K.Westesen, A.S.Ulrich
Novel lipid-nanotubes in dispersions of DMPC
Chem. Phys. Chem. 2004; 5: 1246-1249
- E.Strandberg, A.S.Ulrich
NMR methods for studying membrane-active antimicrobial peptides
Concepts Magn. Res. 2004; 23A: 89-120
- S.Grage, S.Afonin, M.Grüne, A.S.Ulrich
Interaction of the fusogenic peptide B18 in its amyloid-state with DMPC bilayers studied by solid-state 2H- and 31P-NMR and DSC
Chem. Phys. Lipids 2004; 132: 65-77
- R. W. Glaser, C. Sachse, U. H. N. Dürr, P. Wadhwani, A.S.Ulrich
Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels
J. Mag. Res. 2004; 168: 153-163
- S.Afonin, U.H.N.Dürr, R.W.Glaser, A.S.Ulrich
"Boomerang"-like insertion of a fusogenic peptide in a lipid membrane revealed by solid state 19F NMR
Mag. Res. Chem. 2004; 42: 195-203
- T.Asakura, K.Suita, T.Kameda, S.Afonin, A.S.Ulrich
Structural role of tyrosine in Bombyx mori silk fibroin, studied by solid-state NMR and molecular mechanics on a model peptide prepared as silk I and II
Mag. Res. Chem. 2004; 42: 258-266
- O.Toke, R. D.O’Connor, T.K.Weldeghiorghis, W.L.Maloy, R.W.Glaser, A.S.Ulrich, J.Schaefer
Structure of (KIAGKIA)3 aggregates in phospholipid bilayers by solid-state NMR.
Biophys. J. 2004; 87: 675-678
- P.Wadhwani, U.L.H.Dürr,S.Afonin, R.W.Glaser, E.Strandberg, P.Tremouilhac, C.Sachse, M.Berditchevskaja, S.Grage, R.Eisenhuth, D.Maisch, A.S.Ulrich
Solid-state 19F-NMR analysis of membrane-active peptides
Book Chapter
- U. Sternberg, R. Witter, A.S.Ulrich
3D Structure elucidation using NMR Chemical Shifts
Ann. Rep. on NMR 2004; 52: 53-104
2003:
- S.Afonin, R.W.Glaser, M.Berditchevskaja, P.Wadhwani, K.H.Gührs, U.Möllmann, A.S.Ulrich
4-Fluoro-phenylglycine as a label for 19F-NMR structure analysis of membrane-associated peptides
Chem. Bio. Chem 2003; 4: 1151-1163
- U. Sternberg, F.-T. Koch, W. Prieß, R. Witter
Crystal Structure Refinements of Cellulose Polymorphs using solid state 13C Chemical Shifts
Cellulose 2003; 10: 189-199
- R.W.Glaser, A.S.Ulrich
Susceptibility corrections in solid-state NMR experiments with macroscopically oriented membrane samples. Part I: Applications
J. Mag. Res. 2003; 164: 104-114
- R.Ulrich, R.W.Glaser, A.S.Ulrich
Susceptibility corrections in solid-state NMR experiments with macroscopically oriented membrane samples. Part II: Theory
J. Mag. Res. 2003; 164: 115-127
- R.Witter, St.Hesse, and U.Sternberg
Powder Pattern Recoupling at 10 kHz Spinning Speed Applied to Cellulose
J. Magn. Reson. 2003; 161: 35-42
2002:
- A.S.Ulrich
Biophysical aspects of using liposomes as delivery vehicles
Bioscience Reports 2002; 22: 129-150
- S.L.Grage, J.Wang, T.A.Cross, A.S.Ulrich
Structure analysis of fluorine-labelled tryptophan side-chains in gramicidin A by solid state 19F-NMR
Biophys. J. 2002; 83: 3336-3350
- T.Asakura, J.Yao, T.Yamane, K.Umemura, A.S.Ulrich
Heterogeneous structure of silk fibers from Bombyx mori resolved by 13C solid-state NMR spectroscopy
J. Am. Chem. Soc. 2002; 124: 8794-8795
- Witter, W. Prieß, U. Sternberg
Chemical Shift Driven Geometry Optimization
J. Comp. Chem. 2002; 23: 298-305
2001:
- J.Salgado, S.L.Grage, L.H.Kondejewski, R.N.McElhany, R.S.Hodges, A.S.Ulrich
Alignment of the antimicrobial β-sheet peptide gramicidin S in membranes: a solid state 19F-NMR study in oriented lipid bilayers
J. Biomol. NMR 2001; 21: 191-208
- J.Salgado, S.L.Grage, L.H.Kondejewski, R.S. Hodges, R.N.McElhany, A.S.Ulrich
Membrane-bound structure and alignment of the antimicrobial β-sheet peptide gramicidin S derived from angular and distance constraints by solid state 19F-NMR
J. Biomol. NMR 2001; : 1-18
- S.L.Grage, J.Salgado, U.H.N.Dürr, S.Afonin, R.W.Glaser, A.S.Ulrich
Solid state 19F-NMR of biomembranes
In "Perspectives on Solid State NMR in Biology" (Eds. S.Kiihne, H.J.M.de Groot), Kluwers Academic Press: 83-91
- Y.Hori, M.Demura, M.Iwadate, A.S.Ulrich, T.Niidome, H.Aoyagi, T.Asakura
Interaction of mastoparan with membranes studied by 1H-NMR in detergent micelles and by solid state 2H- and 15N-NMR in oriented lipid bilayers
Eur. J. Biochem. 2001; 268: 302-309
- H.Binder, K.Arnold, A.S.Ulrich O.Zschörnig
Interaction of Zn2+ with phospholipid membranes
Biophys. Chem. 2001; 90: 57-74
- T.Heinze, A.Koschella, L.Magdaleno, A.S.Ulrich
Nucleophilic displacement reactions on tosyl cellulose by chiral amines
Polymer Bull. 2001; 46: 7-132000:
- A.S.Ulrich
High resolution 1H and 19F solid state NMR
In "Encyclopedia of Spectroscopy and Spectrometry" (Eds. J.Lindon, G.Tranter, J.Holmes), Academic Press 2000: 813-825
- S.L.Grage, A.S.Ulrich
Orientation-dependent 19F dipolar couplings within a trifluoromethyl-group are revealed by multipulse solid state NMR
J. Mag. Res. 2000; 146: 81-88
- H.Binder, K.Arnold, A.S.Ulrich, O.Zschörnig
The effect of Zn2+ on the secondary structure of a histidine-rich fusogenic peptide and its interaction with lipid membranes
Biochim. Biophys. Acta 2000; 1468: 345-358
- S.L.Grage, D.Gauger, C.Selle, W.Pohle, W.Richter, A.S.Ulrich
The amphiphilic drug flufenamic acid can induce hexagonal lipid phases in DMPC - a 31P and 19F-NMR study
Phys. Chem. Chem. Phys. 2000; 2: 4574-4579
- H.W.Meyer, K.Semmler, W.Rettig, W.Pohle, A.S.Ulrich, S.L.Grage, C.Selle, F.Richter, P.Quinn
Hydration of DMPC and DPPC at 4°C produces a novel gel phase with convex-concave bilayer curvatures
Chem. Phys. Lipids 2000; 105: 149-1661999:
- S.L.Grage, A.S.Ulrich
Structural parameters from 19F homonuclear dipolar couplings, obtained by multipulse solid state NMR in static oriented systems
J. Mag. Res. 1999; 138: 98-106
- A.S.Ulrich, W.Tichelaar, G.Förster, O.Zschörnig, S.Weinkauf, H.W.Meyer
Ultrastructural characterization of peptide-induced membrane fusion and peptide self-assembly in the bilayer
Biophys. J. 1999; 77: 829-841
- R.W.Glaser, M.Grüne, C.Wandelt, A.S.Ulrich
NMR and CD structural analysis of the fusogenic peptide sequence B18 from the fertilization protein bindin
Biochemistry 1999; 38: 2560-2569
- T.Kameda, Y.Ohkawa, K.Yoshizawa, J.Naito, A.S.Ulrich, T.Asakura
Hydrogen-bonding in serine side chains of Bombyx mori and Samia cynthia ricinisilk fibroins using solid state 2H-NMR
Macromol. 1999; 32: 7166-7171
- T.Kameda, Y.Ohkawa, K.Yoshizawa, J.Nakano, A.S.Ulrich, T.Asakura
Dynamics of the tyrosine side chains of Bombyx mori and Samia cynthia ricinisilk fibroins using solid state 2H-NMR
Macromol. 1999; 32: 8491-8495
- H.W.Meyer, H.Bunjes, A.S.Ulrich
Morphological transitions of brain sphingomyelin are determined by the hydration protocol: ripples re-arrange in plane, and sponge-like networks disintegrate into small vesicles
Chem. Phys. Lipids 1999; 99: 111-123
- A.S.Ulrich, A.Watts
Solid state 2H-NMR of lipids and proteins, complemented by neutron diffraction
In "Deuteration of Biological Macromolecules" (Ed. I.N.Serdyuk), JINR Publishing 1999: 57-60
- A.S.Ulrich
Wissenschaft als Beruf
Forschung & Lehre 1999; 5: 238-2411998:
- A.S.Ulrich, S.L.Grage.
In "Solid state NMR on polymers" (Eds. I.Ando, T.Asakura), Elsevier, Amsterdam1998: 190-211
- A.S.Ulrich, M.Otter, C.Glabe, D.Hoekstra
Membrane fusion is triggered by a distinct peptide sequence of the sea urchin fertilization protein bindin
J. Biol. Chem. 1998; 273: 16748-16755
- H.W.Meyer, M.Westermann, W.Richter, M.Stumpf, A.S.Ulrich, C.Hoischen
Minimal radius of curvature of lipid bilayers in the gel state corresponds to the dimension of bimembrane structures "cavaeolae"
J. Struct. Biol. 1998; 124: 77-87
- A.S.Ulrich, S.L.Grage, M.Demura, T.Asakura
2H-Labelling of silk fibroin fibers and their structural characterization by solid state 2H-NMR
In "Internl. Symposium on Silk and NMR" 1998: 35-41
- A.S.Ulrich
Membranfusion
Chemie Heute 98/99: 113-1151997:
- H.Patzelt, A.S.Ulrich,P.Düx, H.Egbringhoff, J.Ashurst, H.Oschkinat, D.Oesterhelt
Towards structural investigations on isotope labelled native bacteriorhodopsin in detergent micelles by solution-state NMR spectroscopy
J. Biomol. NMR 1997; 10: 95-106
- T.Asakura, M.Minami, R.Shimada, M.Demura, M.Osanai, T.Fujito, M.Imanari, A.S.Ulrich
2H-labeling of silk fibroin fibers and their structural characterization by solid-state 2H NMR
Macromol. 1997; 30: 2429-2435
1996:
- A.Loidl-Stahlhofen, A.S.Ulrich, S.Kaufmann, T.M.Bayerl
Protein binding to lipid bilayers controlled by the lipid phase state and mixing behaviour: a new concept for highly selective protein purification
Eur. Biophys. J. 1996; 25: 151-153
- A.S.Ulrich
2H-NMR studies of oriented membranes to determine single bond orientations
Macromol. Symp. 1996; 101: 81-89
- M.Demura, M.Minami, T.Asakura, A.S.Ulrich
Dynamics conformation of uniaxially orientated silk fibroin fiber studied with solid state 2H-NMR
Prog. Polymer Physics 1996; 39: 1-4until 1995:
- Ulrich, A.S., I.Wallat, M.P. Heyn, A.Watts
Re-alignment of the retinal chromophore in the M-state of bacteriorhodopsin
Nature Struct. Biol. 1995; 2: 190-192
- Watts, A., A.S.Ulrich, D.A.Middleton
Solid-state NMR studies of membrane proteins (Review)
Mol. Membr. Biol. 1995; 12: 233-246
- Ulrich, A.S., A.Watts, I.Wallat, M.P.Heyn
Distorted structure of the retinal chromophore in bacteriorhodopsin resolved by 2H-NMR
Biochem. 1994; 33: 5370-5375
- Ulrich, A.S., A.Watts
Molecular response of the lipid headgroup to bilayer hydration as monitored by 2H-NMR
Biophys. J. 1994; 66: 1441-1449
- Ulrich, A.S., A.Watts
Lipid headgroup hydration studied by 2H-NMR: a link between spectroscopy and thermodynamics
Biophys. Chem. 1994; 49: 39-50
- Ulrich, A.S., M.Sami, A.Watts
Hydration of DOPC bilayers by differential scanning calorimetry
Biochim. Biophys. Acta 1994; 1191: 225-230
- Watts, A., B.Sternberg, A.S.Ulrich, C.J.Whiteway, G.Seifert, M.Sami, P.Fisher, M.P.Heyn, I.Wallat
Bacteriorhodopsin: the effect of bilayer thickness on 2D-array formation, and the structural re-alignment of retinal through the photocycle
Biophys. Chem. 1993; 56: 41-46
- Ulrich, A.S., A.Watts
2H-NMR lineshapes of immobilized uniaxially oriented membrane proteins
Solid State NMR 1993; 2: 21-36
- Ulrich, A.S., M.P. Heyn, A.Watts
Structure determination of the cyclohexene-ring of retinal in bacteriorhodopsin by solid-state deuterium-NMR
Biochem. 1992; 31: 10390-10399
- Ulrich, A.S., I.Wallat, M.P.Heyn, A.Watts
Evidence for a curved retinal chain in bacteriorhodopsin from solid-state deuterium-NMR
In "Structures and Functions of Retinal Proteins" (ed. J.L. Rigaud), John Libbey Eurotext 1992: 247-250
- Ulrich, A.S., A.Watts
Membrane protein structure determination by deuterium-NMR
In "Structure, Biogenesis and Dynamics of Biological Membranes" (ed. J.A.F. Op den Kamp), NATO series 1991; Vol. H 63: 243-249
- Ulrich, A.S., F.Volke, A.Watts
The dependence of phospholipid headgroup mobility on hydration asstudied by 2H-NMR spin lattice relaxation time measurements
Chem. Phys. Lipids 1990; 55: 61-66
- Ulrich, A.S., T.Poile, A.Watts
Deuterium NMR to study the surface of phospholipid bilayers
Bull. Magn. Reson. 1990; 12: 80-83
- Blake-Coleman, B.C., A.S.Ulrich, P.Fitzpatrick, M.R.Calder, D.J.Clarke
Apparatus for the electrical characterization of conductive fluids
Biosensors 1989; 4: 87-108

 M. Brehm, S. Heissler, S. Afonin, P.A. Levkin. Nanomolar synthesis in droplet microarrays with UV-triggered on-chip cell screening. Small 1905971 (2020)
M. Brehm, S. Heissler, S. Afonin, P.A. Levkin. Nanomolar synthesis in droplet microarrays with UV-triggered on-chip cell screening. Small 1905971 (2020) M. Drouin, P. Wadhwani, S.L. Grage, J. Bürck, J. Reichert, S. Tremblay, M.S. Mayer, C. Diel, A. Staub, J.-F. Paquin, A.S. Ulrich. Monofluoroalkene-isostere as a 19F-NMR label for the peptide backbone: synthesis and evaluation in membrane-bound PGLa and (KIGAKI)3. Chemistry Eur. J. 26, 1511-1517 (2020)
M. Drouin, P. Wadhwani, S.L. Grage, J. Bürck, J. Reichert, S. Tremblay, M.S. Mayer, C. Diel, A. Staub, J.-F. Paquin, A.S. Ulrich. Monofluoroalkene-isostere as a 19F-NMR label for the peptide backbone: synthesis and evaluation in membrane-bound PGLa and (KIGAKI)3. Chemistry Eur. J. 26, 1511-1517 (2020) R. Sharma, T. C. Asmara, K. Rani Sahoo, S. L. Grage, R. Zhang, J. Sun, S. Das, A. S. Ulrich, A. Rusydi, S. Aryasomayajula, R. Paulmurugan, D. Liepmann, D. Sakthi Kumar, P. Somasundaran, V. Renugopalakrishnan, T. N. Narayanan. Structural and Electronic Transport Properties of Fluorographene Directly Grown on Silicates for Possible Biosensor Applications. ACS Appl. Nano Mater. 3, 5399–5409 (2020)
R. Sharma, T. C. Asmara, K. Rani Sahoo, S. L. Grage, R. Zhang, J. Sun, S. Das, A. S. Ulrich, A. Rusydi, S. Aryasomayajula, R. Paulmurugan, D. Liepmann, D. Sakthi Kumar, P. Somasundaran, V. Renugopalakrishnan, T. N. Narayanan. Structural and Electronic Transport Properties of Fluorographene Directly Grown on Silicates for Possible Biosensor Applications. ACS Appl. Nano Mater. 3, 5399–5409 (2020) Z. You, M. Behl, S.L. Grage, J. Bürck, Q. Zhao, A.S. Ulrich, A. Lendlein. Shape-memory effect by sequential coupling of functions over different length scales in an architectured hydrogel. Biomacromolecules 21, 680-687 (2020)
Z. You, M. Behl, S.L. Grage, J. Bürck, Q. Zhao, A.S. Ulrich, A. Lendlein. Shape-memory effect by sequential coupling of functions over different length scales in an architectured hydrogel. Biomacromolecules 21, 680-687 (2020) P.-A. Paquet-Côté, M. Fillion, M.-È. Provencher, F. Otis, J. Dionne, S. Cardinal, B. Collignon, J. Bürck, P. Lagüe, A.S. Ulrich, M. Auger, N. Voyer. Crown ether modified peptide interactions with model membranes. Supramol. Chem. 31, 159-171 (2019)
P.-A. Paquet-Côté, M. Fillion, M.-È. Provencher, F. Otis, J. Dionne, S. Cardinal, B. Collignon, J. Bürck, P. Lagüe, A.S. Ulrich, M. Auger, N. Voyer. Crown ether modified peptide interactions with model membranes. Supramol. Chem. 31, 159-171 (2019) A. Raasakka, S. Ruskamo, J. Kowal, H. Han, A. Baumann, M. Myllykoski, A. Fasano, R. Rossano, P. Riccio, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Molecular structure and function of myelin protein P0 in membrane stacking. Sci. Rep. 9:642 (2019)
A. Raasakka, S. Ruskamo, J. Kowal, H. Han, A. Baumann, M. Myllykoski, A. Fasano, R. Rossano, P. Riccio, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Molecular structure and function of myelin protein P0 in membrane stacking. Sci. Rep. 9:642 (2019) V. Kubyshkin, S.L. Grage, J. Bürck, A.S. Ulrich, N. Budisa. Transmembrane polyproline helix. J. Phys. Chem. Lett. 9, 2170–2174 (2018)
V. Kubyshkin, S.L. Grage, J. Bürck, A.S. Ulrich, N. Budisa. Transmembrane polyproline helix. J. Phys. Chem. Lett. 9, 2170–2174 (2018) O. Babii, S. Afonin, A.Y. Ishchenko, T. Schober, A.O. Negelia, G.M. Tolstanova, L.V. Garmanchuk, L.I. Ostapchenko, I.V. Komarov, A.S. Ulrich. Structure-activity relationships of photoswitchable diarylethene-based β-hairpin peptides as membranolytic antimicrobial and anticancer agents. J. Med. Chem. 61, 10793-10813 (2018)
O. Babii, S. Afonin, A.Y. Ishchenko, T. Schober, A.O. Negelia, G.M. Tolstanova, L.V. Garmanchuk, L.I. Ostapchenko, I.V. Komarov, A.S. Ulrich. Structure-activity relationships of photoswitchable diarylethene-based β-hairpin peptides as membranolytic antimicrobial and anticancer agents. J. Med. Chem. 61, 10793-10813 (2018) S. Reißer, E. Strandberg, T. Steinbrecher, M. Elstner, A.S. Ulrich. Best of two worlds? How MD simulations of amphiphilic helical peptides in membranes can complement data from oriented solid-state NMR. J. Chem. Theory Comput. 14, 6002-6014 (2018)
S. Reißer, E. Strandberg, T. Steinbrecher, M. Elstner, A.S. Ulrich. Best of two worlds? How MD simulations of amphiphilic helical peptides in membranes can complement data from oriented solid-state NMR. J. Chem. Theory Comput. 14, 6002-6014 (2018) S.L. Grage, S. Kara, A. Bordessa, V. Doan, F. Rizzolo, M. Putzu, T. Kubař, A.M. Papini, G. Chaume, T. Brigaud, S. Afonin, A.S. Ulrich. Orthogonal 19F-labeling for solid-state NMR spectroscopy reveals the conformation and orientation of short peptaibols in membranes. Chemistry Eur. J. 24, 4328–4335 (2018)
S.L. Grage, S. Kara, A. Bordessa, V. Doan, F. Rizzolo, M. Putzu, T. Kubař, A.M. Papini, G. Chaume, T. Brigaud, S. Afonin, A.S. Ulrich. Orthogonal 19F-labeling for solid-state NMR spectroscopy reveals the conformation and orientation of short peptaibols in membranes. Chemistry Eur. J. 24, 4328–4335 (2018) I.V. Komarov, S. Afonin, O. Babii, T. Schober, A.S. Ulrich. Efficiently photocontrollable or not? Biological activity of photoisomerizable diarylethenes. Chemistry Eur. J. 24, 11245-11254 (2018)
I.V. Komarov, S. Afonin, O. Babii, T. Schober, A.S. Ulrich. Efficiently photocontrollable or not? Biological activity of photoisomerizable diarylethenes. Chemistry Eur. J. 24, 11245-11254 (2018) H. Zamora-Carreras, B. Maestro, E. Strandberg, A.S. Ulrich, J.M. Sanz, M.Á. Jiménez. Roles of amphipathicity and hydrophobicity in the micelle-driven structural switch of a 14-mer peptide core from a choline-binding repeat. Chemistry Eur. J. 24, 5825–5839 (2018)
H. Zamora-Carreras, B. Maestro, E. Strandberg, A.S. Ulrich, J.M. Sanz, M.Á. Jiménez. Roles of amphipathicity and hydrophobicity in the micelle-driven structural switch of a 14-mer peptide core from a choline-binding repeat. Chemistry Eur. J. 24, 5825–5839 (2018) E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Bürck, F. Rabanal, A.S. Ulrich. Helix fraying and lipid-dependent structure of a short amphipathic membrane-bound peptide revealed by solid-state NMR. J. Phys. Chem. B 122, 6236-6250 (2018)
E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Bürck, F. Rabanal, A.S. Ulrich. Helix fraying and lipid-dependent structure of a short amphipathic membrane-bound peptide revealed by solid-state NMR. J. Phys. Chem. B 122, 6236-6250 (2018) P. Ridone, S.L. Grage, A. Patkunarajah, A.R. Battle, A.S. Ulrich, B. Martinac. "Force-from-lipids" gating of mechanosensitive channels modulated by PUFAs. J. Mech. Beh. Biomed. Mat. 79, 158-167 (2018)
P. Ridone, S.L. Grage, A. Patkunarajah, A.R. Battle, A.S. Ulrich, B. Martinac. "Force-from-lipids" gating of mechanosensitive channels modulated by PUFAs. J. Mech. Beh. Biomed. Mat. 79, 158-167 (2018) M.-C. Gagnon, E. Strandberg, A.S. Ulrich, J.-F. Paquin, M. Auger. New insights into the influence of monofluorination on dimyristoylphosphatidylcholine membrane properties: A solid-state NMR study. Biochim. Biophys. Acta - Biomembranes 1860, 654-663 (2018)
M.-C. Gagnon, E. Strandberg, A.S. Ulrich, J.-F. Paquin, M. Auger. New insights into the influence of monofluorination on dimyristoylphosphatidylcholine membrane properties: A solid-state NMR study. Biochim. Biophys. Acta - Biomembranes 1860, 654-663 (2018) A.V. Strizhak, K. Sharma, O. Babii, S. Afonin, A.S. Ulrich, I.V. Komarov, D.R. Spring. Highly reactive bis-cyclooctyne-modified diarylethene for SPAAC-mediated cross-linking. Org. Biomol. Chem. 16, 8559–8564 (2018)
A.V. Strizhak, K. Sharma, O. Babii, S. Afonin, A.S. Ulrich, I.V. Komarov, D.R. Spring. Highly reactive bis-cyclooctyne-modified diarylethene for SPAAC-mediated cross-linking. Org. Biomol. Chem. 16, 8559–8564 (2018) O.M. Michurin, K. Tolmachova, S. Afonin, O. Babii, S.L. Grage, A.S. Ulrich, I.V. Komarov, D.S. Radchenko. Conformationally constrained mono-fluorinated arginine as a cationic label for solid-state 19F NMR analysis of membrane-bound peptides. Eur. J. Org. Chem. 2018, 3826–3833 (2018)
O.M. Michurin, K. Tolmachova, S. Afonin, O. Babii, S.L. Grage, A.S. Ulrich, I.V. Komarov, D.S. Radchenko. Conformationally constrained mono-fluorinated arginine as a cationic label for solid-state 19F NMR analysis of membrane-bound peptides. Eur. J. Org. Chem. 2018, 3826–3833 (2018) J. Bürck, O. Aras, L. Bertinetti, C.A. Ilhan, M.A. Ermeydan, R. Schneider, A.S. Ulrich, M. Kazanci. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 1151, 73-80 (2018)
J. Bürck, O. Aras, L. Bertinetti, C.A. Ilhan, M.A. Ermeydan, R. Schneider, A.S. Ulrich, M. Kazanci. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 1151, 73-80 (2018) S. Reißer, S. Prock, H. Heinzmann, A.S. Ulrich. Protein ORIGAMI: A program for the creation of 3D paper models of folded peptides. Biochem. Mol. Biol. Education 46, 403–409 (2018)
S. Reißer, S. Prock, H. Heinzmann, A.S. Ulrich. Protein ORIGAMI: A program for the creation of 3D paper models of folded peptides. Biochem. Mol. Biol. Education 46, 403–409 (2018) S. Afonin, V. Kubyshkin, P.K. Mykhailiuk, I.V. Komarov, A.S. Ulrich. Conformational plasticity of the cell-penetrating peptide SAP as revealed by solid-state 19F-NMR and circular dichroism spectroscopies. J. Phys. Chem. B 121, 6479-6491 (2017)
S. Afonin, V. Kubyshkin, P.K. Mykhailiuk, I.V. Komarov, A.S. Ulrich. Conformational plasticity of the cell-penetrating peptide SAP as revealed by solid-state 19F-NMR and circular dichroism spectroscopies. J. Phys. Chem. B 121, 6479-6491 (2017) ober, I.V. Komarov, A.S. Ulrich. Flexibility vs rigidity of amphipathic peptide conjugates when interacting with lipid bilayers. Biochim. Biophys. Acta 1859, 2505-2515 (2017)
ober, I.V. Komarov, A.S. Ulrich. Flexibility vs rigidity of amphipathic peptide conjugates when interacting with lipid bilayers. Biochim. Biophys. Acta 1859, 2505-2515 (2017) M. Berditsch, M. Trapp, S. Afonin, C. Weber, J. Misiewicz, J. Turkson, A.S. Ulrich. Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens. Sci. Rep. 7, 44324 (2017)
M. Berditsch, M. Trapp, S. Afonin, C. Weber, J. Misiewicz, J. Turkson, A.S. Ulrich. Antimicrobial peptide gramicidin S is accumulated in granules of producer cells for storage of bacterial phosphagens. Sci. Rep. 7, 44324 (2017) M.-C. Gagnon, E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Reichert, J. Bürck, F. Rabanal, M. Auger, J.-F. Paquin, A.S. Ulrich. Influence of length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochem. 56, 1680−1695 (2017) DOI: 10.1021/acs.biochem.6b01071
M.-C. Gagnon, E. Strandberg, A. Grau-Campistany, P. Wadhwani, J. Reichert, J. Bürck, F. Rabanal, M. Auger, J.-F. Paquin, A.S. Ulrich. Influence of length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochem. 56, 1680−1695 (2017) DOI: 10.1021/acs.biochem.6b01071 S.L. Grage, M.-A. Sani, O. Cheneval, S. Troeira Henriques, C. Schalck, R. Heinzmann, J.S. Mylne, P.K. Mykhailiuk, S. Afonin, I.V. Komarov, F. Separovic, D.J. Craik, A.S. Ulrich. Orientation and location of the cyclotide kalata B1 in phospholipid bilayers revealed by solid-state NMR. Biophys. J. 112, 630-642 (2017)
S.L. Grage, M.-A. Sani, O. Cheneval, S. Troeira Henriques, C. Schalck, R. Heinzmann, J.S. Mylne, P.K. Mykhailiuk, S. Afonin, I.V. Komarov, F. Separovic, D.J. Craik, A.S. Ulrich. Orientation and location of the cyclotide kalata B1 in phospholipid bilayers revealed by solid-state NMR. Biophys. J. 112, 630-642 (2017) S. Kara, S. Afonin, O. Babii, A.N. Tkachenko, I.V. Komarov, A.S. Ulrich. Diphytanoyl lipids as model systems for studying membrane-active peptides. Biochim. Biophys. Acta 1859, 1828-1837 (2017)
S. Kara, S. Afonin, O. Babii, A.N. Tkachenko, I.V. Komarov, A.S. Ulrich. Diphytanoyl lipids as model systems for studying membrane-active peptides. Biochim. Biophys. Acta 1859, 1828-1837 (2017) M.J. Klein, S. Schmidt, P. Wadhwani, J. Bürck, J.Reichert, S. Afonin, M. Berditsch, T. Schober, R. Brock, M. Kansy, A.S. Ulrich. Lactam-stapled cell-penetrating peptides: Cell uptake and membrane binding properties. J. Med. Chem. 60, 8071-8082 (2017)
M.J. Klein, S. Schmidt, P. Wadhwani, J. Bürck, J.Reichert, S. Afonin, M. Berditsch, T. Schober, R. Brock, M. Kansy, A.S. Ulrich. Lactam-stapled cell-penetrating peptides: Cell uptake and membrane binding properties. J. Med. Chem. 60, 8071-8082 (2017) L. Li, M. Schmitt, A. Matzke-Ogi, P. Wadhwani, V. Orian-Rousseau, P.A. Levkin. CD44v6-peptide functionalized nanoparticles selectively bind to metastatic cancer cells. Adv. Sci. 4, 1600202 (2017)
L. Li, M. Schmitt, A. Matzke-Ogi, P. Wadhwani, V. Orian-Rousseau, P.A. Levkin. CD44v6-peptide functionalized nanoparticles selectively bind to metastatic cancer cells. Adv. Sci. 4, 1600202 (2017) P. Mühlhäuser, P. Wadhwani, E. Strandberg, J. Bürck, A.S. Ulrich. Structure analysis of the membrane-bound DCD-derived peptide SSL-25 from human sweat. Biochim. Biophys. Acta 1859, 2308-2318 (2017)
P. Mühlhäuser, P. Wadhwani, E. Strandberg, J. Bürck, A.S. Ulrich. Structure analysis of the membrane-bound DCD-derived peptide SSL-25 from human sweat. Biochim. Biophys. Acta 1859, 2308-2318 (2017) T. Murayama, T. Masuda, S. Afonin, K. Kawano, T. Takatani-Nakase, H. Ida, Y. Takahashi, T. Fukuma, A.S. Ulrich, S. Futaki. Loosening of lipid packing promotes oligoarginine entry into cells. Angew. Chem. Int. Ed. 56, 7644-7647 (2017)
T. Murayama, T. Masuda, S. Afonin, K. Kawano, T. Takatani-Nakase, H. Ida, Y. Takahashi, T. Fukuma, A.S. Ulrich, S. Futaki. Loosening of lipid packing promotes oligoarginine entry into cells. Angew. Chem. Int. Ed. 56, 7644-7647 (2017) C. Priem, A. Wuttke, M. Berditsch, A.S. Ulrich, A. Geyer. Scaling the amphiphilic character and antimicrobial activity of Gramicidin S by dihydroxylation or ketal formation. J. Org. Chem. in press (2017)
C. Priem, A. Wuttke, M. Berditsch, A.S. Ulrich, A. Geyer. Scaling the amphiphilic character and antimicrobial activity of Gramicidin S by dihydroxylation or ketal formation. J. Org. Chem. in press (2017) M. Putzu, S. Kara, S. Afonin, S.L. Grage, A. Bordessa, G. Chaume, T. Brigaud , A.S. Ulrich, T. Kubař. Structural behavior of the peptaibol Harzianin HK VI in a DMPC bilayer: Insights from MD simulations. Biophys. J. 112, 2602-2614 (2017)
M. Putzu, S. Kara, S. Afonin, S.L. Grage, A. Bordessa, G. Chaume, T. Brigaud , A.S. Ulrich, T. Kubař. Structural behavior of the peptaibol Harzianin HK VI in a DMPC bilayer: Insights from MD simulations. Biophys. J. 112, 2602-2614 (2017) A. Raasakka, S. Ruskamo, J. Kowal, R. Barker, A. Baumann, A. Martel, J. Tuusa, M. Myllykoski, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Sci. Rep. 7: 4974 (2017)
A. Raasakka, S. Ruskamo, J. Kowal, R. Barker, A. Baumann, A. Martel, J. Tuusa, M. Myllykoski, J. Bürck, A.S. Ulrich, H. Stahlberg, P. Kursula. Membrane association landscape of myelin basic protein portrays formation of the myelin major dense line. Sci. Rep. 7: 4974 (2017) E. Strandberg, A.S. Ulrich. Solid-state 19F-NMR analysis of peptides in oriented biomembranes. In: Modern Magnetic Resonance, pages 1-18. (Springer International Publishing, 2017, ed. Graham A. Webb)
E. Strandberg, A.S. Ulrich. Solid-state 19F-NMR analysis of peptides in oriented biomembranes. In: Modern Magnetic Resonance, pages 1-18. (Springer International Publishing, 2017, ed. Graham A. Webb) E. Strandberg, A.S. Ulrich (2017). Solid-state NMR for studying peptide structures and peptide-lipid interactions in membranes. In: Modern Magnetic Resonance, pages 1-13. (Springer International Publishing, 2017, ed. Graham A. Webb)
E. Strandberg, A.S. Ulrich (2017). Solid-state NMR for studying peptide structures and peptide-lipid interactions in membranes. In: Modern Magnetic Resonance, pages 1-13. (Springer International Publishing, 2017, ed. Graham A. Webb) H. Turgut, A.C. Schmidt, P. Wadhwani, A. Welle, R. Müller, G. Delaittre. The para-fluoro-thiol ligation in water. Polymer Chem. 8, 1288-1293 (2017)
H. Turgut, A.C. Schmidt, P. Wadhwani, A. Welle, R. Müller, G. Delaittre. The para-fluoro-thiol ligation in water. Polymer Chem. 8, 1288-1293 (2017) A. Turshatov, D. Busko, N. Kiseleva, S.L. Grage, I.A. Howard, B.S. Richards. Room-temperature high-efficiency solid-state triplet-triplet annihilation up-conversion in amorphous poly(olefin sulfone)s. ACS Appl. Mat. Interf. 9, 8280-8286 (2017)
A. Turshatov, D. Busko, N. Kiseleva, S.L. Grage, I.A. Howard, B.S. Richards. Room-temperature high-efficiency solid-state triplet-triplet annihilation up-conversion in amorphous poly(olefin sulfone)s. ACS Appl. Mat. Interf. 9, 8280-8286 (2017) J. Zerweck, E. Strandberg, O. Kukharenko, J. Reichert, J. Bürck, P. Wadhwani, A.S. Ulrich. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep.
J. Zerweck, E. Strandberg, O. Kukharenko, J. Reichert, J. Bürck, P. Wadhwani, A.S. Ulrich. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep.  P. Wadhwani, N. Heidenreich, B. Podeyn, J. Bürck, A.S. Ulrich. Antibiotic gold: tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater. Sci. 5, 817-827 (2017)
P. Wadhwani, N. Heidenreich, B. Podeyn, J. Bürck, A.S. Ulrich. Antibiotic gold: tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater. Sci. 5, 817-827 (2017) R. Wallbrecher, T. Ackels, R.A. Olea, M.J. Klein, L. Caillon, J. Schiller, P.H. Bovée-Geurts, T.H. van Kuppevelt, A.S. Ulrich, M. Spehr, M.J.W. Adjobo-Hermans, R. Brock. Membrane permeation of arginine-rich cell-penetrating peptides independent of transmembrane potential as a function of lipid composition and membrane fluidity. J. Contr. Release 256, 68-78 (2017)
R. Wallbrecher, T. Ackels, R.A. Olea, M.J. Klein, L. Caillon, J. Schiller, P.H. Bovée-Geurts, T.H. van Kuppevelt, A.S. Ulrich, M. Spehr, M.J.W. Adjobo-Hermans, R. Brock. Membrane permeation of arginine-rich cell-penetrating peptides independent of transmembrane potential as a function of lipid composition and membrane fluidity. J. Contr. Release 256, 68-78 (2017) T. Asakura, T. Ohata, S. Kametani, K. Okushita, K. Yazawa, Y. Nishiyama, K. Nishimura, A. Aoki, F. Sizuki, H. Kaji, A. S. Ulrich, M. P. Williamson. Intermolecular packing in B. mori silk fibroin: Multinuclear NMR study of the model peptide (Ala-Gly)15 defines a heterogeneous antiparallel antipolar mode of assembly in the silk II form. Macromolecules 48, 28-36 (2015)
T. Asakura, T. Ohata, S. Kametani, K. Okushita, K. Yazawa, Y. Nishiyama, K. Nishimura, A. Aoki, F. Sizuki, H. Kaji, A. S. Ulrich, M. P. Williamson. Intermolecular packing in B. mori silk fibroin: Multinuclear NMR study of the model peptide (Ala-Gly)15 defines a heterogeneous antiparallel antipolar mode of assembly in the silk II form. Macromolecules 48, 28-36 (2015) D. Bandak, O. Babii, R. Vasiuta, I. V. Komarov, P. K. Mykhailiuk. Design and synthesis of novel 19F-amino acid: A promising 19F NMR label for peptide studies. Org. Lett. 17, 226-229 (2015)
D. Bandak, O. Babii, R. Vasiuta, I. V. Komarov, P. K. Mykhailiuk. Design and synthesis of novel 19F-amino acid: A promising 19F NMR label for peptide studies. Org. Lett. 17, 226-229 (2015) M. Berditsch, S. Afonin, A. Steineker, N. Orel, I. Jakovkin, C. Weber and A. S. Ulrich. Fermentation and cost-effective 13C/15N labeling of the nonribosomal peptide gramicidin S for nuclear magnetic resonance structure analysis. Appl. Environ. Microbiol. 81, 3593-3603 (2015)
M. Berditsch, S. Afonin, A. Steineker, N. Orel, I. Jakovkin, C. Weber and A. S. Ulrich. Fermentation and cost-effective 13C/15N labeling of the nonribosomal peptide gramicidin S for nuclear magnetic resonance structure analysis. Appl. Environ. Microbiol. 81, 3593-3603 (2015) M. Berditsch, T. Jäger, N. Strempel, T. Schwartz, J. Overhage and A. S. Ulrich. Synergistic effect of membrane active peptides polymyxin B and gramicidin S on multidrug resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy 59, 5288-5296 (2015)
M. Berditsch, T. Jäger, N. Strempel, T. Schwartz, J. Overhage and A. S. Ulrich. Synergistic effect of membrane active peptides polymyxin B and gramicidin S on multidrug resistant strains and biofilms of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy 59, 5288-5296 (2015) D. S. Blokhin, A. V. Filippov, O. N. Antzutkin, S. Afonin and V. V. Klochkov. Spatial structures of PAP(262 270) and PAP(274 284), two selected fragments of PAP(248 286), an enhancer of HIV infectivity. Appl. Magn. Reson. 46, 757-769 (2015)
D. S. Blokhin, A. V. Filippov, O. N. Antzutkin, S. Afonin and V. V. Klochkov. Spatial structures of PAP(262 270) and PAP(274 284), two selected fragments of PAP(248 286), an enhancer of HIV infectivity. Appl. Magn. Reson. 46, 757-769 (2015) J. Bürck, S. Roth, D. Windisch, P. Wadhwani, D. Moss and A. S. Ulrich. UV-CD12: synchrotron radiation circular dichroism beamline at ANKA. Journal of Synchrotron Radiation 22, 844-852 (2015)
J. Bürck, S. Roth, D. Windisch, P. Wadhwani, D. Moss and A. S. Ulrich. UV-CD12: synchrotron radiation circular dichroism beamline at ANKA. Journal of Synchrotron Radiation 22, 844-852 (2015) M. Cakici, Z.-G. Gu, M. Nieger, J. Bürck, L. Heinke and S. Bräse. Planar-chiral building blocks for metal-organic frameworks. Chem. Comm. 51, 4796-4798 (2015)
M. Cakici, Z.-G. Gu, M. Nieger, J. Bürck, L. Heinke and S. Bräse. Planar-chiral building blocks for metal-organic frameworks. Chem. Comm. 51, 4796-4798 (2015) V. Kubyshkin, S. Afonin, S. Kara, N. Budisa, P. K. Mykhailiuk and A. S. Ulrich. gamma-(S)-Trifluoromethyl proline: Evaluation as a structural substitute of proline for solid state 19F-NMR peptide studies. Org. Biomol. Chem. 13, 3171-3181 (2015)
V. Kubyshkin, S. Afonin, S. Kara, N. Budisa, P. K. Mykhailiuk and A. S. Ulrich. gamma-(S)-Trifluoromethyl proline: Evaluation as a structural substitute of proline for solid state 19F-NMR peptide studies. Org. Biomol. Chem. 13, 3171-3181 (2015) G. Manzo, M. A. Scorciapino, P. Wadhwani, J. Bürck, N. P. Montaldo, M. Pintus, R. Sanna, M. Casu, A. Giuliani, G. Pirri, V. Luca, A. S. Ulrich and A. C. Rinaldi. Enhanced amphiphilic profile of a short ß-stranded peptide improves its antimicrobial activity. PLoS One 10, e0116379 (2015)
G. Manzo, M. A. Scorciapino, P. Wadhwani, J. Bürck, N. P. Montaldo, M. Pintus, R. Sanna, M. Casu, A. Giuliani, G. Pirri, V. Luca, A. S. Ulrich and A. C. Rinaldi. Enhanced amphiphilic profile of a short ß-stranded peptide improves its antimicrobial activity. PLoS One 10, e0116379 (2015) J. Misiewicz, S. Afonin, S. L. Grage, J. van den Berg, E. Strandberg, P. Wadhwani and A. S. Ulrich. Action of the multifunctional peptide BP100 on native biomembranes examined by solid-state NMR. J. Biomol. NMR 61, 287-298 (2015)
J. Misiewicz, S. Afonin, S. L. Grage, J. van den Berg, E. Strandberg, P. Wadhwani and A. S. Ulrich. Action of the multifunctional peptide BP100 on native biomembranes examined by solid-state NMR. J. Biomol. NMR 61, 287-298 (2015) J. Misiewicz, S. Afonin and A. S. Ulrich. Control and role of pH in peptide-lipid interactions in oriented membrane samples. Biochim. Biophys. Acta - Biomembranes 1848, 833-841 (2015)
J. Misiewicz, S. Afonin and A. S. Ulrich. Control and role of pH in peptide-lipid interactions in oriented membrane samples. Biochim. Biophys. Acta - Biomembranes 1848, 833-841 (2015)
 T. Serdiuk, I. Bakanovich, V. Lysenko, S. A. Alekseev, V. A. Skryshevsky, S. Afonin, E. Berger, A. Geloen and I. V. Komarov. Delivery of SiC-based nanoparticles into live cells driven by cell-penetrating peptides SAP and SAP-E. RSC Advances 5, 20498-20502 (2015)
T. Serdiuk, I. Bakanovich, V. Lysenko, S. A. Alekseev, V. A. Skryshevsky, S. Afonin, E. Berger, A. Geloen and I. V. Komarov. Delivery of SiC-based nanoparticles into live cells driven by cell-penetrating peptides SAP and SAP-E. RSC Advances 5, 20498-20502 (2015) B. A. Wallace, J. Bürck. Third international synchrotron radiation circular dichroism spectroscopy meeting. Synchrotron Radiation News 28, 58-59 (2015)
B. A. Wallace, J. Bürck. Third international synchrotron radiation circular dichroism spectroscopy meeting. Synchrotron Radiation News 28, 58-59 (2015) D. Windisch, C. Ziegler, S. L. Grage, J. Bürck, M. Zeitler, P. L. Gor'kov, A. S. Ulrich. Hydrophobic mismatch drives the interaction of E5 with the transmembrane segment of PDGF receptor. Biophys. J. 109, 737-749 (2015)
D. Windisch, C. Ziegler, S. L. Grage, J. Bürck, M. Zeitler, P. L. Gor'kov, A. S. Ulrich. Hydrophobic mismatch drives the interaction of E5 with the transmembrane segment of PDGF receptor. Biophys. J. 109, 737-749 (2015)